
Immune checkpoint inhibitors combined with paclitaxel-based chemotherapy versus chemotherapy alone as first-line treatment in HER2-negative advanced gastric cancer: result of a multicenter retrospective study
Highlight box
Key findings
• Paclitaxel-based chemotherapy plus immune checkpoint inhibitors (ICIs) showed significantly improved progression-free survival, overall survival, duration of response, objective response rate & disease control rate compared to chemotherapy alone in first-line therapy of advanced gastric cancer (AGC), with tolerable side effects.
What is known and what is new?
• Platinum-based chemotherapy plus ICIs is the standard first-line treatment for human epidermal growth factor receptor 2 (HER2)-negative AGC, while paclitaxel-based therapy has shown good efficacy and tolerability in AGC as an alternative regimen for first-line chemotherapy of AGC in China.
• Paclitaxel-based chemotherapy plus ICIs is superior to chemotherapy alone in efficacy for first-line therapy of AGC.
What is the implication, and what should change now?
• ICIs combined with paclitaxel-based chemotherapy should be an alternative regimen in first-line treatment for HER2-negative AGC, and large prospective trials are warranted to confirm our findings.
Introduction
Background
Gastric cancer is one of the most common tumors in the world, especially in East Asian countries (1). Unfortunately, most of the patients have unresectable tumors at their initial consultation in China, resulting in a poor overall prognosis (2). Currently, for advanced gastric cancer (AGC), the basic form of treatment is still palliative chemotherapy but it shows limited survival advantage (3). Median survival for patients using chemotherapy alone is generally less than 1 year (4). Despite the proliferation of new drugs, effective therapies to lengthen patients’ survival with AGC are still lacking.
Rationale and knowledge gap
Recently, newly developed immune checkpoint inhibitors (ICIs) have shown reliable anti-cancer effects in many solid tumors, and ICIs have become the standard of care treatment for serval malignancies (5-7). For the treatment of AGC, some of the latest pivotal phase III studies of programmed cell death-1 (PD-1) inhibitors along with conventional agents demonstrated encouraging efficacy. These findings have changed the first-line therapy landscape for AGC. In CheckMate-649, nivolumab plus chemotherapy (FOLFOX/XELOX) significantly improved overall survival (OS) in the programmed cell death ligand-1 (PD-L1) combined positive score (CPS) ≥5 group and overall population. In the PD-L1 CPS ≥5 group, nivolumab combined with FOLFOX/XELOX resulted in a median OS of 14.4 months and a median progression-free survival (PFS) of 7.7 months, which exceeded 6.1 months and 11.1 months compared with chemotherapy alone (8). Nivolumab plus oxaliplatin-based chemotherapy has been approved by the Food and Drug Administration (FDA) as the first-line therapy for AGC irrespective of PD-L1 CPS based on the findings of CheckMate-649. In China, guidelines list nivolumab plus XELOX/FOLFOX the preferred regimen for PD-L1-positive AGC (9). Additionally, in China, many newly developed domestic ICIs are used in clinical trials for first-line treatment of AGC, such as ORIENT-16 (10), CS1001-101 (11) and SHR1210 (12). The above research results have confirmed the exact efficacy of paclitaxel plus ICIs in the therapy of AGC.
Currently, platinum-based chemotherapy is the standard regimen for first-line therapy of AGC (13,14). However, platinum-based regimen typically has more toxic events (15). Paclitaxel alone or in combination with fluoropyrimidine shows good efficacy and tolerability in AGC, and is considered an alternative to first-line chemotherapy for AGC in China (16,17). In a previous phase III clinical study, paclitaxel-based chemotherapy demonstrated a more favorable survival benefit and more manageable toxicity compared to oxaliplatin plus S-1, further proving the feasibility of paclitaxel as first-line treatment (18). Furthermore, studies have shown that paclitaxel may enhance the function of antigen-presenting cells (APCs), inhibiting the function of regulatory T cells (Tregs) and modulating related cells to promote the activity of cytotoxic T cells, thus enhancing anti-tumor immune response and improving the efficacy of immunotherapy (19-22). Meanwhile, the efficacy of paclitaxel combined with immunotherapy is supported by several studies in neoadjuvant or conversion therapy and second-line therapy of AGC (23,24).
Objective
Until now, immunotherapy plus paclitaxel-based chemotherapy as first-line therapy has not been reported. Thus, the objective of our retrospective research was to compare the efficacy and safety of immunotherapy plus paclitaxel-based chemotherapy as a first-line therapy in AGC versus paclitaxel-based chemotherapy alone. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-814/rc).
Methods
Patients
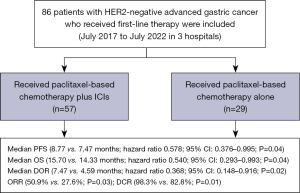
In this retrospective research, we compared the efficacy and safety of ICIs in combination with paclitaxel plus fluoropyrimidine (the ICIs plus chemotherapy group) versus paclitaxel plus fluoropyrimidine (the chemotherapy alone group). Our study collected data from eighty-six AGC patients receiving first-line treatment from July 2017 to July 2022 at three hospitals (Zhejiang Cancer Hospital, Taizhou Cancer Hospital and Zhejiang Medical & Health Group Hangzhou Hospital) in China. Among 86 patients, 57 were included in the ICIs plus chemotherapy group and 29 were included in the chemotherapy alone group. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committees of Zhejiang Cancer Hospital (No. IRB-2022-443), Taizhou Cancer Hospital (No. 2023-031), and Zhejiang Medical & Health Group Hangzhou Hospital (No. 2023015). Because of the retrospective nature of the study, the requirement for informed consent was waived. The inclusion criteria include: (I) gastric adenocarcinoma confirmed by histology; (II) human epidermal growth factor receptor 2 (HER2)-negative; (III) previously untreated metastatic or unresectable disease (If the time of recurrence was greater than 6 months from the last adjuvant chemotherapy, prior adjuvant therapy was allowed); (IV) measurable lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1; (V) Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0, 1 or 2; (VI) patients received at least two cycles of therapy. Key exclusion criteria included active gastrointestinal bleeding, unsolved digestive tract obstruction, inadequate hepatic or renal function, loss to follow-up and incomplete treatment data.
Treatment
The ICIs plus chemotherapy group received ICIs combined with paclitaxel-based chemotherapy. The paclitaxel-based chemotherapy included paclitaxel (75 mg/m2 intravenously on day 1 & 8) or nab-paclitaxel (125 mg/m2 intravenously on day 1 & 8) in combination with capecitabine (1,000 mg/m2 bid d1–14) or S-1 (40 mg/m2 bid d1–14). Chemotherapy was administered every three weeks. Patients received anti-PD-1 antibody 200 mg/time which was repeated every three weeks or as instructed. The chemotherapy group received paclitaxel-based chemotherapy alone. After receiving up to eight cycles of combination therapy, patients who remained stable disease (SD) were then administered capecitabine or S-1 with anti-PD-1 antibody as maintenance treatment. Treatments were terminated when the disease progressed, or when the patient experienced intolerable adverse effects, or patients expressed consent for withdrawal.
Assessments
Tumor imaging was evaluated at baseline and after every two or three cycles according to RECIST v1.1. OS was the period from the date of first receipt of antineoplastic therapy to death for any reason. PFS was the period from the date of first receipt of antineoplastic therapy to first documented tumor progression or death for any reason before progression. Duration of response (DOR) was the period from the first tumor assessment as partial response (PR) or complete response (CR) to the first assessment as progressive disease (PD) or death from any cause before PD. Objective response rate (ORR) was the proportion of patients whose tumors achieved response (PR and CR) during treatment. Disease control rate (DCR) was the proportion of patients whose tumors achieved CR, PR and SD during treatment. Criteria for censoring included: (I) no disease progression or death by May 2023, (II) patient loss to follow-up. The censoring time of this subset patients was May 2023 or the date of last follow-up. Safety was assessed by the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Statistical analysis
The t-tests and Chi-squared test (χ2 test) were utilized to examine differences in baseline characteristics. OS and PFS curves were estimated by Kaplan-Meier method and compared between the groups by the log-rank test. The hazard ratio (HR) was calculated using the Cox proportional hazards model. Statistics were considered significant for P values <0.05. The SPSS program, version 22.0, was used to analyze all of the data.
Results
Patient characteristics
After excluding 29 ineligible patients (8 cases only received one cycle of anti-tumor therapy, 12 cases tested positive for HER2, 6 cases were lost to follow-up and 3 cases had incomplete treatment data) from the available cohort, we finally included 86 patients in this study. Among them, 57 cases were included in the ICIs plus chemotherapy group, and 29 cases were included in the chemotherapy alone group. Disease characteristics at baseline are shown in Table 1. The ICIs plus chemotherapy group tended to include more patients with higher ECOG PS scores and patients with liver metastases (24.6% vs. 6.9%).
Table 1
| Characteristics | ICI plus chemotherapy (n=57) | Chemotherapy alone (n=29) | P value |
|---|---|---|---|
| Age (years), mean ± SD | 59.09±12.87 | 55.17±11.04 | 0.16 |
| Sex, n (%) | 0.91 | ||
| Male | 38 (66.7) | 19 (65.5) | |
| Female | 19 (33.3) | 10 (34.5) | |
| ECOG PS, n (%) | 0.04* | ||
| 0 | 34 (59.6) | 25 (86.2) | |
| 1 | 22 (38.6) | 4 (13.8) | |
| 2 | 1 (1.8) | 0 | |
| Primary tumor location, n (%) | 0.12 | ||
| Cardia | 9 (15.8) | 1 (3.4) | |
| Gastric funds | 4 (7.0) | 2 (6.9) | |
| Gastric body | 17 (29.8) | 10 (34.5) | |
| Gastric antrum | 21 (36.8) | 16 (55.2) | |
| Multiple/diffuse | 6 (10.5) | 0 (0.0) | |
| Histology, n (%) | 0.12 | ||
| Well differentiated | 0 (0) | 0 (0) | |
| Moderately differentiated | 11 (19.3) | 2 (6.9) | |
| Poorly differentiated | 46 (80.7) | 27 (93.1) | |
| Metastatic site, n (%) | |||
| Peritoneum | 30 (52.6) | 16 (55.2) | 0.82 |
| Liver | 14 (24.6) | 2 (6.9) | 0.04* |
| Lymph node | 25 (43.9) | 12 (41.4) | 0.82 |
| Ovary | 6 (10.5) | 4 (13.8) | 0.65 |
| Others | 14 (24.6) | 5 (17.2) | 0.43 |
| PD-L1 status, n (%) | 0.32 | ||
| ≥1 | 13 (22.8) | 3 (10.3) | |
| <1 | 5 (8.8) | 2 (6.9) | |
| Unknown | 39 (68.4) | 24 (68.4) | |
| MMR, n (%) | 0.77 | ||
| pMMR/MSS | 31 (54.4) | 15 (51.7) | |
| dMMR/MSI-H | 2 (3.5) | 2 (6.9) | |
| Unknown | 24 (42.1) | 12 (41.4) | |
| EBER, n (%) | 0.08 | ||
| Positive | 3 (5.3) | 0 | |
| Negative | 17 (29.8) | 15 (51.7) | |
| Unknown | 37 (64.9) | 14 (48.3) | |
| Chemotherapy regimens, n (%) | 0.22 | ||
| P/A | 9 (15.8) | 3 (10.3) | |
| PS/AS | 42 (73.3) | 19 (65.5) | |
| PX/AX | 6 (10.5) | 7 (24.1) | |
| ICIs regimens, n (%) | – | ||
| Sintilimab | 36 (63.2) | – | |
| Nivolumab | 7 (12.3) | – | |
| Tislelizumab | 6 (10.5) | – | |
| Camrelizumab | 4 (7.0) | – | |
| Toripalimab | 4 (7.0) | – | |
*, represents P<0.05. ICI, immune checkpoint inhibitor; SD, standard deviation; ECOG PS, Eastern Cooperative Oncology Group performance status; PD-L1, programmed cell death ligand 1; MMR, mismatch repair; pMMR, proficient MMR; MSS, microsatellite stable; dMMR, deficient MMR; MSI-H, microsatellite instability-high; EBER, Epstein-Barr encoding region; P, paclitaxel alone; A, nab-paclitaxel alone; PS, paclitaxel plus S-1; AS, nab-paclitaxel plus S-1; PX, paclitaxel plus capecitabine; AX, nab-paclitaxel plus capecitabine.
The median follow-up was 25.37 months and median treatment cycle was seven and six cycles in two groups, respectively. In the ICIs plus chemotherapy group, the ICIs used were sintilimab (36 patients, 63.2%), nivolumab (7 patients, 12.3%), tislelizumab (6 patients, 10.5%), camrelizumab (4 patients, 7.0%) and toripalimab (4 patients, 7.0%). For chemotherapy regimen in the ICIs plus chemotherapy group, paclitaxel (3 patients), nab-paclitaxel (7 patients), paclitaxel plus S-1 (21 patients), paclitaxel plus capecitabine (1 patient), nab-paclitaxel plus S-1 (20 patients), and nab-paclitaxel plus capecitabine (5 patients) were used. Meanwhile, in the chemotherapy alone group, paclitaxel (2 patients), nab-paclitaxel (1 patient), paclitaxel plus S-1 (11 patients), paclitaxel plus capecitabine (3 patients), nab-paclitaxel plus S-1 (8 patients), and nab-paclitaxel plus capecitabine (4 patients) were used (Table 1). In the chemotherapy alone group population, 21 (72.4%) of 29 patients received subsequent anticancer therapy, including 18 (62.1%) patients who received ICIs therapies. In the ICIs plus chemotherapy group, 29 (50.9%) patients received subsequent anticancer therapy, 17 (29.8%) of whom went on to receive anti-PD-1 therapy.
Efficacy
In the ICIs plus chemotherapy group, 1 (1.8%) of the 57 patients had CR, 28 (49.1%) showed PR, 27 (47.4%) achieved SD, and 1 (1.8%) progressed. In the chemotherapy alone group, 8 (27.6%) of the 29 patients showed PR, 16 (55.2%) had SD, and 5 (17.2%) progressed. The ICIs plus chemotherapy group significantly increased ORR (50.9% vs. 27.6%; P=0.03) and DCR (98.3% vs. 82.8%; P=0.01) compared with the chemotherapy alone group (Table 2).
Table 2
| Tumor response | ICIs plus chemotherapy (n=57) | Chemotherapy alone (n=29) | P value* |
|---|---|---|---|
| Complete response | 1 | 0 | |
| Partial response | 28 | 8 | |
| Stable disease | 27 | 16 | |
| Progressive disease | 1 | 5 | |
| Objective response | 29 (50.9%; 37.5–64.3%) | 8 (27.6%; 10.3–44.9%) | 0.03 |
| Disease control | 56 (98.3%; 94.7–101.8%) | 24 (82.8%; 68.1–97.4%) | 0.01 |
Data are presented as n or n (%; 95% CI). *, P value for χ2 test. ICIs plus chemotherapy, combination therapy of anti-PD-1 antibody and paclitaxel-based chemotherapy; Chemotherapy alone, therapy of paclitaxel-based chemotherapy. RECIST, Response Evaluation Criteria in Solid Tumors; ICIs, immune checkpoint inhibitors; CI, confidence interval.
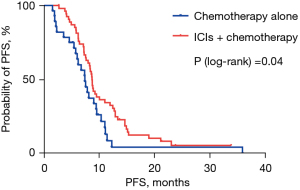
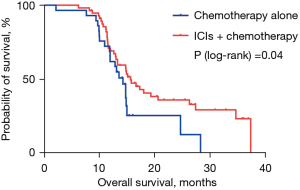
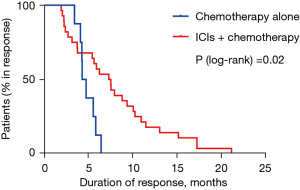
The median PFS was 8.77 months in the ICIs plus chemotherapy group and 7.47 months in the chemotherapy alone group (HR 0.578; 95% CI: 0.376–0.995; P=0.04; Figure 1). The 1-year PFS rates in two groups were 32.6% and 4.4% respectively. Median OS was 15.70 months in the ICIs plus chemotherapy group and 14.33 months in the chemotherapy alone group (HR 0.540; 95% CI: 0.293–0.993; P=0.04; Figure 2). The 1-year survival rates in two groups were 69.3% and 65.5%, and the 2-year survival rates were 36.2% and 12.8% respectively. Median DOR was 7.47 months in the ICIs plus chemotherapy group and 4.59 months in the chemotherapy alone group (HR 0.368; 95% CI: 0.148–0.916; P=0.02; Figure 3). In the ICIs plus chemotherapy group, significant prolongation of PFS, OS and DOR were observed.
Adverse events (AEs)
AEs in both groups of patients under treatment are listed in Tables 3,4. In sum, the regimens were well tolerated by patients in both groups, and most adverse reactions were grade 2 or lower. Bone marrow suppression, gastrointestinal reactions and alopecia were common in two groups (incidence >50%). The incidences of rash, abnormal liver function and hypothyroidism were higher in the ICIs plus paclitaxel-based chemotherapy group, but there was no statistical difference. Rash and hypothyroidism were thought to have a connection to ICIs. Bone marrow suppression (incidence >10%) was the most common severe AEs. There was no difference in the incidence of grade 3–4 AEs between the two groups.
Table 3
| AEs | ICIs plus chemotherapy | Chemotherapy alone | P value |
|---|---|---|---|
| Bone marrow suppression | 43 (75.4) | 22 (75.9) | 0.96 |
| Neurotoxicity | 8 (14.0) | 6 (20.7) | 0.42 |
| Nausea and vomiting | 34 (59.6) | 15 (51.7) | 0.48 |
| Diarrhea | 14 (25.0) | 6 (20.7) | 0.65 |
| Hand-foot syndrome | 7 (12.3) | 6 (20.7) | 0.30 |
| Fatigue | 26 (45.6) | 11 (37.9) | 0.49 |
| Alopecia | 36 (63.2) | 19 (65.5) | 0.82 |
| Rash | 16 (28.1) | 3 (10.3) | 0.06 |
| Abnormal liver function | 11 (19.3) | 2 (6.9) | 0.23 |
| Hypothyroidism | 5 (8.8) | 0 | 0.24 |
Data are presented as n (%). AEs, adverse events; ICIs, immune checkpoint inhibitors.
Table 4
| AEs | ICIs plus chemotherapy | Chemotherapy alone | P value |
|---|---|---|---|
| Bone marrow suppression | 10 (17.5) | 6 (20.7) | 0.72 |
| Neurotoxicity | 1 (1.8) | 1 (3.4) | 0.62 |
| Nausea and vomiting | 4 (7.0) | 1 (3.4) | 0.50 |
| Diarrhea | 2 (3.5) | 0 | 0.30 |
| Hand-foot syndrome | 2 (3.5) | 2 (6.9) | 0.48 |
| Fatigue | 0 | 0 | – |
| Alopecia | 0 | 0 | – |
| Rash | 3 (5.3) | 0 | 0.20 |
| Abnormal liver function | 0 | 1 (3.4) | 0.33 |
| Hypothyroidism | 0 | 0 | – |
Data are presented as n (%). AEs, adverse events; ICIs, immune checkpoint inhibitors.
Discussion
Key findings
This is the first multicenter, retrospective research to explore the efficacy and tolerability of ICIs plus paclitaxel-based chemotherapy as the first-line therapy of AGC. The results of our study demonstrated that the median PFS (8.77 versus 7.47 months; P=0.04), median OS (15.70 versus 14.33 months; P=0.04) and DOR (7.47 versus 4.59 months; P=0.02) in the ICIs plus paclitaxel-based chemotherapy group were significantly prolonged. Meanwhile, the ORR (50.9% vs. 27.6%; P=0.03) and DCR (98.3% vs. 82.8%; P=0.01) were also significantly increased in the ICIs plus chemotherapy group (Figure 4). Most patients may continue receiving medication until disease progressed since the toxicity was manageable.
Strengths and limitations
This is the first multicenter, retrospective research to explore the efficacy and tolerability of ICIs plus paclitaxel-based chemotherapy as the first-line therapy of AGC. Paclitaxel is an alkaloid extracted from the Taxus brevifolia. Because of its unique anticancer mechanism and broad anticancer activity, paclitaxel is currently considered to be one of the most popular and effective natural drugs (25,26). Nab-paclitaxel is a paclitaxel in the albumin-bound from of nanoparticles, devoid of any solvents or ethanol. Studies have shown comparable efficacy of nab-paclitaxel and ordinary paclitaxel in the treatment of AGC, so both types of paclitaxel were included in our study (27,28). Our results showed for the first time that paclitaxel-based chemotherapy combined with ICIs resulted in higher ORR as well as longer PFS and OS compared with chemotherapy alone, with manageable adverse effects. Our results demonstrated the feasibility of paclitaxel in combination with ICIs for the treatment of advanced first-line gastric cancer and may provide patients with a highly effective therapeutic option with manageable side effects.
However, this research also had some limitations as a retrospective study. First, due to the successful application of ICIs in the therapy of gastric cancer, fewer patients received paclitaxel chemotherapy alone, only 29 patients were enrolled between 2019 and 2021. The sample size was small and not well balanced in two groups. Second, as efficacy indicators of immunotherapy, expression of PD-L1, mismatch repair (MMR), and Epstein-Barr encoding region (EBER) were not routinely detected for the diagnosis of gastric cancer in our hospital before 2021, thus the expression status of these three biomarkers in the two groups of patients were not completely known. Third, because some patients still did not reach the survival endpoint, final survival data were not collected for all patients in this study, which may affect the results of the final analysis.
Comparison with similar researches
For first-line treatment, previous phase III studies have confirmed the definite efficacy and good tolerability of paclitaxel/nab-paclitaxel plus fluoropyrimidine. Recently, the result of GAPSO study showed that ACG patients received AS (nab-paclitaxel plus S-1) tended to have better PFS (9.03 vs. 5.07 months; P=0.03) than those treated with SOX with acceptable toxicities as the first-line therapy of AGC. A phase III study initiated by Lu et al. compared the efficacy of paclitaxel plus capecitabine versus cisplatin plus capecitabine, the results showed similar median PFS (5.0 vs. 5.3 months; P=0.44) and median OS (12.5 vs. 11.8 months; P=0.30) in two arms (29). ORR (43.1 vs. 28.8%; P=0.01) was improved in paclitaxel plus capecitabine arm versus cisplatin plus capecitabine arm. The results of our study demonstrated a median PFS of 8.77 months in the ICIs plus chemotherapy group and a median PFS of 7.47 months in the chemotherapy alone group. Our PFS is similar to the results of phase II/III clinical studies in recent years but longer than that of Lu’s study. This may be related to the general conditions of enrolled patients and the increase of treatment options in recent years.
Explanations of findings
Through blocking the binding of immune checkpoint molecules to the ligands, ICIs deregulate the immunosuppressive effect of PD-1, expose tumor cells to the immune activity of effector T cells and generate tumor immune response (30). Combining ICIs with other anti-cancer modalities including chemotherapy may result in better immunological conditions which could promote the cancer therapy efficiency (31). Paclitaxel exerts neoplastic effects by disrupting microtubules (32). Paclitaxel is a well-known immunogenic cell death inducer, many studies have demonstrated that paclitaxel as an immunogenic cell death inducer can modulate a variety of immune cells. Paclitaxel can activate cytotoxic T lymphocytes, while inhibiting the growth and function of immune-suppressive cells, such as tumor-associated fibroblasts, Tregs, and myeloid-derived suppressor cells (20-22). A study showed that nanomicelle-encapsulated paclitaxel (nano-paclitaxel) stimulates the infiltration of CD8+ T lymphocytes by inducing immunologic cell death (33). However, tumor-infiltrating CD8+ T cells with high PD-1 expression and upregulation of PD-L1 by both immune cells and tumor cells hinder this antitumor immunity following nano-paclitaxel treatment. Interestingly, it was found that nano-paclitaxel combined with anti-PD-1 antibody can induce CD8+ T cell-dependent anti-cancer immunity and effectively promote the therapeutic effect by paclitaxel. In addition, when compared to other chemotherapeutic medications like cisplatin or oxaliplatin, combined treatment with nano-paclitaxel and anti-PD-1 antibodies significantly promotes tumor regression and demonstrates a considerable improvement in the survival of mice.
Clinically, paclitaxel combined with immunotherapy has been used in the treatment of a variety of malignancies (34-36). And several previous trials have shown the feasibility of paclitaxel plus ICIs. The KEYNOTE-407 trial demonstrated significantly longer OS and PFS in squamous cell carcinoma patients who received paclitaxel/nab-paclitaxel plus pembrolizumab (37). The IMpower130 study demonstrated that atezolizumab combined with nab-paclitaxel plus carboplatin as first-line therapy for NSCLC significantly prolonged median OS and median PFS compared with chemotherapy alone (38). In addition, a study demonstrated the evidence that paclitaxel plus ICIs was beneficial and safe in patients with refractory melanoma (36). In the treatment of AGC, the efficacy of paclitaxel combined with immunotherapy has been initially proved in neoadjuvant or translational therapy and second-line treatment. The CO-STAR study evaluated the surgical conversion feasibility of sintilimab in combination with nab-paclitaxel, S-1 and apatinib for the conversion therapy of AGC. Of 36 evaluable patients, ORR was 61.1% and the R0 surgical conversion rate was 47.2% (23). Nivolumab in combination with paclitaxel plus ramucirumab was used as the second-line therapy of AGC in another phase I/II trial (UMIN-CTR). The results showed a median OS of 13.1 months for the combination regimen (39). The above research results all prove the reliable efficacy and acceptable tolerability of paclitaxel combined with immunotherapy in the treatment of AGC.
Implications and actions needed
Based on the evidence mentioned above, we conducted this retrospective study to investigate the efficacy and safety of ICIs plus paclitaxel-based chemotherapy in the first-line therapy of AGC. Our findings showed that ICIs combined with paclitaxel-based chemotherapy should be an alternative regimen in first-line treatment for HER2-negative AGC. This is the first retrospective study to prove that ICIs combined with paclitaxel have good efficacy in the first-line treatment of AGC, and may provide a new option for the first-line treatment of AGC. Large prospective trials are warranted to confirm our findings in the future.
Conclusions
In conclusion, ICIs combined with paclitaxel-based chemotherapy should be an alternative regimen in first-line treatment for HER2-negative AGC, and large prospective trials are warranted to confirm our findings.
Acknowledgments
Funding: This study was sponsored by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-814/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-814/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-814/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-814/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committees of Zhejiang Cancer Hospital (No. IRB-2022-443), Taizhou Cancer Hospital (No. 2023-031), and Zhejiang Medical & Health Group Hangzhou Hospital (No. 2023015). Because of the retrospective nature of the study, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Qi J, Li M, Wang L, et al. National and subnational trends in cancer burden in China, 2005-20: an analysis of national mortality surveillance data. Lancet Public Health 2023;8:e943-55. [Crossref] [PubMed]
- Patel TH, Cecchini M. Targeted Therapies in Advanced Gastric Cancer. Curr Treat Options Oncol 2020;21:70. [Crossref] [PubMed]
- Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006;24:2903-9. [Crossref] [PubMed]
- Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet 2021;398:1002-14. [Crossref] [PubMed]
- Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016;17:976-83. [Crossref] [PubMed]
- Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 2019;20:1239-51. [Crossref] [PubMed]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
- Wang FH, Zhang XT, Li YF, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond) 2021;41:747-95. [Crossref] [PubMed]
- Xu J, Jiang H, Pan Y, et al. Sintilimab Plus Chemotherapy for Unresectable Gastric or Gastroesophageal Junction Cancer: The ORIENT-16 Randomized Clinical Trial. JAMA 2023;330:2064-74. [Crossref] [PubMed]
- Shen L, Li J, Miao Z, et al. 1445P CS1001, an anti-PD-L1 antibody, combined with standard of care (SOC) chemotherapy for first line (1L) advanced GC/GEJ and ESCC: Preliminary results from 2 phase Ib cohorts of CS1001-101 study. Ann Oncol 2020;31:S909. [Crossref]
- Xu J, Zhang Y, Jia R, et al. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clin Cancer Res 2019;25:515-23. [Crossref] [PubMed]
- Lordick F, Carneiro F, Cascinu S, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33:1005-20. [Crossref] [PubMed]
- Lu J, Ding Y, Chen Y, et al. Whole-exome sequencing of alpha-fetoprotein producing gastric carcinoma reveals genomic profile and therapeutic targets. Nat Commun 2021;12:3946. [Crossref] [PubMed]
- Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008;9:215-21. [Crossref] [PubMed]
- Sakamoto J, Matsui T, Kodera Y. Paclitaxel chemotherapy for the treatment of gastric cancer. Gastric Cancer 2009;12:69-78. [Crossref] [PubMed]
- Waddell T, Verheij M, Allum W, et al. Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi57-63. [Crossref] [PubMed]
- Dai YH, Yu XJ, Xu HT, et al. Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study). Ther Adv Med Oncol 2022;14:17588359221118020. [Crossref] [PubMed]
- Coleman S, Clayton A, Mason MD, et al. Recovery of CD8+ T-cell function during systemic chemotherapy in advanced ovarian cancer. Cancer Res 2005;65:7000-6. [Crossref] [PubMed]
- Huang D, Yang Y, Zhang S, et al. Regulatory T-cell density and cytotoxic T lymphocyte density are associated with complete response to neoadjuvant paclitaxel and carboplatin chemoradiotherapy in gastric cancer. Exp Ther Med 2018;16:3813-20. [Crossref] [PubMed]
- Michels T, Shurin GV, Naiditch H, et al. Paclitaxel promotes differentiation of myeloid-derived suppressor cells into dendritic cells in vitro in a TLR4-independent manner. J Immunotoxicol 2012;9:292-300. [Crossref] [PubMed]
- Zhang L, Dermawan K, Jin M, et al. Differential impairment of regulatory T cells rather than effector T cells by paclitaxel-based chemotherapy. Clin Immunol 2008;129:219-29. [Crossref] [PubMed]
- Jandova J, Wondrak GT. Vemurafenib Drives Epithelial-to-Mesenchymal Transition Gene Expression in BRAF Inhibitor-Resistant BRAFV600E/NRASQ61K Melanoma Enhancing Tumor Growth and Metastasis in a Bioluminescent Murine Model. J Invest Dermatol 2022;142:1456-65.e1. [Crossref] [PubMed]
- Wang J, He Y, Zhang B, et al. The Efficacy and Safety of Sintilimab Combined With Nab-Paclitaxel as a Second-Line Treatment for Advanced or Metastatic Gastric Cancer and Gastroesophageal Junction Cancer. Front Oncol 2022;12:924149. [Crossref] [PubMed]
- Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007;357:2666-76. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Shitara K, Takashima A, Fujitani K, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol 2017;2:277-87. [Crossref] [PubMed]
- Ishikawa M, Iwasa S, Nagashima K, et al. Retrospective comparison of nab-paclitaxel plus ramucirumab and paclitaxel plus ramucirumab as second-line treatment for advanced gastric cancer focusing on peritoneal metastasis. Invest New Drugs 2020;38:533-40. [Crossref] [PubMed]
- Lu Z, Zhang X, Liu W, et al. A multicenter, randomized trial comparing efficacy and safety of paclitaxel/capecitabine and cisplatin/capecitabine in advanced gastric cancer. Gastric Cancer 2018;21:782-91. [Crossref] [PubMed]
- Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol 2021;16:223-49. [Crossref] [PubMed]
- Heinhuis KM, Ros W, Kok M, et al. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol 2019;30:219-35. [Crossref] [PubMed]
- Swanton C, Marani M, Pardo O, et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell 2007;11:498-512. [Crossref] [PubMed]
- Yang Q, Shi G, Chen X, et al. Nanomicelle protects the immune activation effects of Paclitaxel and sensitizes tumors to anti-PD-1 Immunotherapy. Theranostics 2020;10:8382-99. [Crossref] [PubMed]
- Vaddepally RK, Kharel P, Pandey R, et al. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers (Basel) 2020;12:738. [Crossref] [PubMed]
- Gravara LD, Battiloro C, Cantile R, et al. Chemotherapy and/or immune checkpoint inhibitors in NSCLC first-line setting: what is the best approach? Lung Cancer Manag 2020;9:LMT22. [Crossref] [PubMed]
- Li JJ, Wang JH, Dingv Y, et al. Efficacy and safety of anti-PD-1 inhibitor combined with nab-paclitaxel in Chinese patients with refractory melanoma. J Cancer Res Clin Oncol 2022;148:1159-69. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:924-37. [Crossref] [PubMed]
- Nakajima TE, Kadowaki S, Minashi K, et al. Multicenter Phase I/II Study of Nivolumab Combined with Paclitaxel Plus Ramucirumab as Second-line Treatment in Patients with Advanced Gastric Cancer. Clin Cancer Res 2021;27:1029-36. [Crossref] [PubMed]


