
PD-L1 expression in pancreaticobiliary adenosquamous carcinoma: a single-institution case series
Highlight box
Key findings
• Programmed cell death ligand 1 (PD-L1) expression is significantly more common in pancreaticobiliary adenosquamous carcinomas (PB-ASCs) than in pancreaticobiliary adenocarcinomas (PB-ACs) (66.7% vs. 8.8%, P<0.0001).
• The immune desert phenotype, characterized by a paucity of lymphocytes in both the tumor parenchyma and the stroma, is less common in PB-ASCs than in PB-ACs (26.7% vs. 64.7%; P=0.02).
What is known and what is new?
• PB-ASC is known to be a highly aggressive tumor with dismal survival outcomes.
• PB-ASCs are notably enriched in inflammatory response and show higher PD-L1 expression than PB-ACs.
What is the implication, and what should change now?
• Relatively high expression of PD-L1 by PB-ASC tumor cells suggests a potential therapeutic role for immune checkpoint inhibitors in managing patients with PB-ASC.
Introduction
Adenosquamous carcinoma (ASC) of the pancreaticobiliary tract (PB-ASC) is a rare subgroup of pancreatic and biliary ductal carcinomas estimated to constitute less than 5% of all pancreaticobiliary malignancies (1,2). Pancreatic ASC is associated with more aggressive clinical and histopathological features compared with pancreatic adenocarcinoma (AC), including poorer differentiation, larger tumor size, and higher rates of node-positive disease (3,4). Among patients who undergo resection, those with pancreatic ASC have a poorer prognosis than those with pancreatic AC, with a median survival of 4–6 months in the former group (2,3). Similarly, ASC of the gallbladder portends worse outcomes than AC of the gallbladder (5-7). In a study of 606 cases of resected invasive gallbladder carcinoma, patients with ASC (n=34) were more likely to have advanced stage (> pT2) and significantly worsened survival outcomes (median survival 11.4 months) compared to those with AC (5). Notably, cases of ASC were often characterized by an abundance of pleomorphic tumor giant cells and tumor-infiltrating eosinophils. Additional studies have further shown that ASC of the extrahepatic bile duct is also regarded as an aggressive tumor with a less favorable prognosis (median survival 6–13 months) than AC of the extrahepatic bile duct (6,7). The poor survival of patients with ASC of the extrahepatic bile duct may be due in part to the association of the disease with aggressive pathological features including: deeper and more frequent duodenal and pancreatic invasion, increased node-positive disease, and higher disease stage at diagnosis (6-8). Even with the knowledge that ASC arising in the pancreaticobiliary tree portends worsened clinical outcomes than AC arising in the same organs, there is little difference in the clinical management of these patients. Rather, the overall rarity of pancreaticobiliary ASC and the scarcity of reported cases continues to hamper our understanding of the clinical, pathologic, and prognostic characteristics of this disease.
The programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) pathway plays a critical role in physiologic inhibition and modulation of the immune response in normal tissue (9-12). PD-L1 is a transmembrane protein expressed on the surface of T-lymphocytes (T-cells), B-lymphocytes, and antigen-presenting cells, and a variety of non-lymphoid tissues. Ligand binding of PD-L1 with PD-1 expressed on cytotoxic T-cells and other immune cells elicits signals that mediate immune tolerance by inhibiting cytotoxic T-cell activity and proliferation while simultaneously reducing apoptosis of regulatory T-cells (9,10,12). It is well-established that PD-1 and its ligand PD-L1 induce immunogenic tolerance in solid and hematological malignancies, where increased levels of tumor-expressed PD-L1 influence the immune response and is a key determinant of checkpoint-immunotherapy efficacy (13-15).
In recent studies of non-small cell lung cancers, PD-L1 expression tended to be higher in squamous cell carcinomas and ASCs than in ACs (16,17). Likewise, PD-L1 expression in lung cancers was significantly higher in the squamous component of ASCs than in the glandular component (18-20). Such increases in PD-L1 expression within the squamous component likely provides these lung cancer cells with an improved means of escaping immune surveillance than tumor cells comprising the glandular component. Similar studies of PD-L1 expression in PB-ASC remain limited and contradictory. Whereas one study found no significant differences in the frequencies or patterns of PD-L1 expression between ASC and AC (21), several others have shown a positive association between PD-L1 and the ASC phenotype (22,23). Notably, increased PD-L1 expression among cases of pancreatic ASC than AC, appears to be largely confined to the squamous component of the ASCs (22,23). Likewise, in gallbladder carcinomas, PD-L1 expression appears more often within the squamous component (24); however, no studies, to date, have examined PD-L1 expression in ASC of the bile duct.
Despite the highly aggressive phenotype and dismal survival associated with PB-ASC, the overall rarity of this disease has limited our ability to differentially treat it from more common AC. To address the urgent and unmet need to develop effective therapeutic strategies against this peculiar subtype of pancreaticobiliary carcinoma, the present study sought to examine PD-L1 expression in relation to tumor-infiltrating lymphocytes (TILs) in PB-ASC and conventional pancreaticobiliary AC (PB-AC). We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-9/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was conducted following Institutional Review Board approval, in the Department of Pathology at the University of North Carolina at Chapel Hill (IRB No. 19-1862). Because of the retrospective nature of the study involving only existing data and human biological specimens, the requirement for informed consent was waived.
Patient and tissue selection
Following a retrospective search of the electronic medical record, this study included 15 patients with PB-ASC (10 pancreatic, 5 gallbladder) and 34 control patients with PB-AC (22 pancreatic ductal, 12 gallbladder) diagnosed between January 1, 2000 and June 1, 2019 at a single university hospital (University of North Carolina at Chapel Hill). No cases of intrahepatic or extrahepatic bile-duct ASC were found in our record and thus, biliary tract carcinomas in this study are virtually limited to gallbladder lesions. Resection specimens were available for 11 of the PB-ASC cases (6 pancreatic, 5 gallbladder), whereas fine needle biopsy (FNB) or fine needle aspiration (FNA) specimens were available for the other four cases of pancreatic ASC. Resection specimens were available for all of the control PB-AC cases. Histological slides of all cases were reviewed by two pathologists (M.F. and E.S.) who confirmed the diagnosis of PB-ASC or PB-AC. Mucicarmine staining and/or immunohistochemical (IHC) staining for p63 and CK5/6 was utilized to confirm the diagnosis of PB-ASC in cases where glandular or squamous differentiation was equivocal on routine histology. One or two representative tissue blocks from each case were used for further analysis. All tissue blocks obtained had sufficient tissue for additional IHC studies.
Immunohistochemistry
Chromogenic IHC was performed on formalin-fixed and paraffin-embedded (FFPE) tissue sections (5-µm thickness) using the Leica Bond III Autostainer system (Leica Biosystems, Deer Park, IL, USA) following the manufacturer’s instructions (25). The details of the primary antibodies used, their dilutions, and the procedure for antigen retrieval are summarized in Table 1. Slides were deparaffinized in Bond Dewax solution (Leica, AR9222) and hydrated in Bond Wash solution (Leica, AR9590). Heat-induced antigen retrieval was performed at 100 ℃ in either Bond-Epitope Retrieval Solution 1 pH 6.0 (Leica, AR9961) or Bond-Epitope Retrieval Solution 2 pH 9.0 (Leica, AR9640). Antigen retrieval was followed by a 5-minute peroxide blocking step (IPB5000L, Biocare Medical, Pacheco, CA, USA), after which slides were incubated with the primary antibody followed by Leica Post Primary and Novolink Polymer (Leica, RE7260-CE) secondary reagents. Antibody detection with 3,3'-diaminobenzidine (DAB) and hematoxylin counterstain was performed using the Bond Intense R detection system (Leica, DS9263). Stained slides were dehydrated and coverslipped with Cytoseal 60 (23-244256, Fisher Scientific, Pittsburgh, PA, USA). Appropriate positive controls were used for each assay.
Table 1
| Antibody | Clone | Cat number, source | Dilution | Primary incubation time | Protocol |
|---|---|---|---|---|---|
| CD3 | LN10 | PA0553, Leica (Deer Park, IL, USA) | Prediluted | 15 min | Bond-Epitope Retrieval Solution 2 |
| CD8 | 4B11 | PA0183, Leica (Deer Park, IL, USA) | Prediluted | 15 min | Bond-Epitope Retrieval Solution 2 |
| CK 5/6 | D5/16B4 | 356M-14, Cell Marque (Rocklin, CA, USA) | 1:100 | 60 min | Bond-Epitope Retrieval Solution 1 |
| P63 | BC4A4 | PM163AA, Biocare Medical (Pacheco, CA, USA) | Prediluted | 15 min | Bond-Epitope Retrieval Solution 2 |
| PD-L1 | E1L3N | 13684, Cell Signaling Technology (Danvers, MA, USA) | 1:50 | 60 min | Bond-Epitope Retrieval Solution 1 |
PD-L1, programmed cell death ligand 1.
Microscopic evaluation
In addition to histologic confirmation of the diagnosis, all tumors were classified into the following three immune phenotypes based on routine histology: (I) immune inflamed (II), characterized by the presence of abundant lymphocytes in the tumor parenchyma; (II) immune excluded (IE), characterized by the presence of abundant lymphocytes in the stroma, but not in the tumor parenchyma; and (III) immune desert (ID), characterized by a paucity of lymphocytes in both the tumor parenchyma and the stroma. In cases of the II phenotype, the presence of abundant CD8-positive T-cells in the tumor parenchyma was confirmed by IHC staining for CD3 and CD8.
IHC staining for PD-L1 was independently evaluated by three individuals (J.D.W., M.F., and E.S.) who each interpreted the IHC expression of PD-L1 semi-quantitatively using a tumor proportion score (TPS), which is the percentage of viable tumor cells showing partial or complete clear membranous staining (26,27). Background cytoplasmic staining and staining of normal cells, necrotic cells, and cellular debris were not counted. Controls were considered appropriate if at least 70% of the stained cells contained convincing membrane staining above background noise. No PD-L1 expression was reported for <1% of viable tumor cells, whereas low PD-L1 expression was reported for 1–49% of viable tumor cells, and high PD-L1 expression was reported for ≥50% of viable tumor cells. At least 100 viable tumor cells were evaluated for the presence or absence of PD-L1 staining in each specimen. For the PB-ASC specimens, TPS was determined separately in the AC and squamous components in areas where the two components were clearly distinguishable. The overall expression status of PD-L1 was compared between PB-ASCs and ACs.
Statistical analysis
Fisher’s exact test and nonparametric Mann-Whitney U test were performed as appropriate using GraphPad Prism version 9.3.1 (GraphPad Software, Boston, Massachusetts, USA). P values <0.05 were considered significant.
Results
Clinicopathologic data is summarized in Table 2. A total of 15 PB-ASC cases (10 pancreatic, 5 gallbladder) and 34 PB-AC cases (22 pancreatic, 12 gallbladder) were identified. The mean age at the time of diagnosis was 65 years (range, 44–92 years). Of the 15 PB-ASC cases, 9 (60%) were male and 6 (40%) were female. Among the 34 PB-AC control cases, 12 (35%) were male and 22 (65%) were female. Demographic characteristics (age, sex) were not statistically different in patients with PB-ASC and those with PB-AC.
Table 2
| Case No. | Age (years) | Sex | Tissue | Histologic type [% of SC] | PD-L1 TPS | Cancer-immune phenotype | ||
|---|---|---|---|---|---|---|---|---|
| Source | Type | AC [%] | SC [%] | |||||
| 1 | 72 | F | Panc | FNB | ASC [80] | Low [5] | 0 | IE |
| 2 | 78 | M | Panc | FNB | ASC [50] | 0 | 0 | ID |
| 3 | 67 | M | Panc | FNB | ASC [60] | 0 | Low [30] | ID |
| 4 | 69 | M | Panc | FNA | ASC [90] | 0 | 0 | IE |
| 5 | 65 | F | Panc | Resection | ASC [50] | Low [1] | Low [30] | IE |
| 6 | 52 | M | Panc | Resection | ASC [60] | 0 | 0 | IE |
| 7 | 72 | M | Panc | Resection | ASC [80] | 0 | Low [5] | IE |
| 8 | 46 | M | Panc | Resection | ASC [40] | High [60] | High [60] | II |
| 9 | 46 | M | Panc | Resection | ASC [25] | 0 | High [60] | IE |
| 10 | 70 | F | Panc | Resection | ASC [40] | Low [20] | Low [30] | ID |
| 11 | 60 | F | Gb | Resection | ASC [60] | 0 | Low [20] | IE |
| 12 | 67 | F | Gb | Resection | ASC [80] | 0 | Low [10] | IE |
| 13 | 92 | M | Gb | Resection | ASC [60] | 0 | 0 | IE |
| 14 | 58 | F | Gb | Resection | ASC [30] | 0 | 0 | ID |
| 15 | 50 | M | Gb | Resection | ASC [40] | 0 | High [60] | II |
| 16 | 46 | M | Panc | Resection | AC [0] | 0 | NA | ID |
| 17 | 57 | F | Panc | Resection | AC [0] | High [60] | NA | IE |
| 18 | 78 | F | Panc | Resection | AC [0] | 0 | NA | IE |
| 19 | 69 | F | Panc | Resection | AC [0] | 0 | NA | ID |
| 20 | 50 | M | Panc | Resection | AC [0] | 0 | NA | IE |
| 21 | 44 | M | Panc | Resection | AC [0] | 0 | NA | ID |
| 22 | 53 | M | Panc | Resection | AC [0] | 0 | NA | ID |
| 23 | 74 | M | Panc | Resection | AC [0] | 0 | NA | ID |
| 24 | 65 | F | Panc | Resection | AC [0] | 0 | NA | ID |
| 25 | 60 | M | Panc | Resection | AC [0] | 0 | NA | ID |
| 26 | 75 | F | Panc | Resection | AC [0] | 0 | NA | ID |
| 27 | 71 | F | Panc | Resection | AC [0] | 0 | NA | ID |
| 28 | 62 | F | Panc | Resection | AC [0] | Low [30] | NA | IE |
| 29 | 66 | M | Panc | Resection | AC [0] | 0 | NA | ID |
| 30 | 61 | F | Panc | Resection | AC [0] | 0 | NA | ID |
| 31 | 75 | F | Panc | Resection | AC [0] | 0 | NA | ID |
| 32 | 64 | F | Panc | Resection | AC [0] | 0 | NA | ID |
| 33 | 53 | M | Panc | Resection | AC [0] | 0 | NA | ID |
| 34 | 72 | F | Panc | Resection | AC [0] | 0 | NA | ID |
| 35 | 75 | M | Panc | Resection | AC [0] | 0 | NA | ID |
| 36 | 77 | M | Panc | Resection | AC [0] | 0 | NA | ID |
| 37 | 70 | F | Panc | Resection | AC [0] | 0 | NA | IE |
| 38 | 64 | F | Gb | Resection | AC [0] | 0 | NA | ID |
| 39 | 70 | F | Gb | Resection | AC [0] | 0 | NA | IE |
| 40 | 74 | F | Gb | Resection | AC [0] | Low [30] | NA | II |
| 41 | 58 | F | Gb | Resection | AC [0] | 0 | NA | IE |
| 42 | 70 | F | Gb | Resection | AC [0] | 0 | NA | ID |
| 43 | 54 | F | Gb | Resection | AC [0] | 0 | NA | ID |
| 44 | 74 | F | Gb | Resection | AC [0] | 0 | NA | IE |
| 45 | 86 | F | Gb | Resection | AC [0] | 0 | NA | ID |
| 46 | 50 | M | Gb | Resection | AC [0] | 0 | NA | IE |
| 47 | 61 | F | Gb | Resection | AC [0] | 0 | NA | IE |
| 48 | 78 | M | Gb | Resection | AC [0] | 0 | NA | ID |
| 49 | 74 | F | Gb | Resection | AC [0] | 0 | NA | IE |
PD-L1, programmed cell death ligand 1; ASC, adenosquamous carcinoma; AC, adenocarcinoma; SC, squamous cell carcinoma; TPS, tumor proportion score; F, female; M, male; Panc, pancreas; Gb, gallbladder; FNB, fine needle biopsy; FNA, fine needle aspiration; IE, immune exclusive; ID, immune desert; II, immune inflamed.
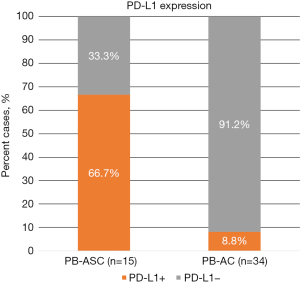
The IHC results for PD-L1 and cancer-immune phenotypes are summarized in Table 2. PD-L1 was expressed in 10 of 15 (66.7%) PB-ASCs compared with 3 of 34 (8.8%) control PB-ACs (P<0.001; Figure 1). The histologic findings of representative cases are shown in Figures 2,3. There was no significant difference in PD-L1 expression between PB-ASCs of pancreatic origin and PB-ASCs of gallbladder origin. In a subpopulation analysis of the ten PB-ASCs with PD-L1 expression, PD-L1 expression was exclusive to the squamous component in six cases, exclusive to the glandular component in one case, and observed in both the squamous component and the glandular component in three cases. There was no significant association between PD-L1 positivity and tumor component (squamous vs. glandular) among the PB-ASCs (P>0.99). Similarly, among the control PB-ACs, there was no significant difference in PD-L1 positivity between ACs of pancreatic origin and ACs of gallbladder origin (P>0.99).
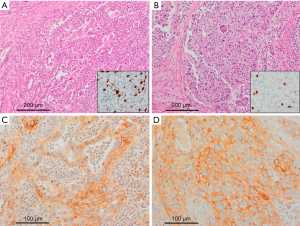

Next, we compared the tumor immune phenotypes between PB-ASC and PB-AC (Table 2 and Figure 4). The II phenotype was present in 2 of 15 (13.3%) PB-ASCs compared with 1 of 34 (2.9%) control PB-ACs, with no significant difference between the two groups (P=0.21). Similarly, the IE phenotype was present in 9 of 15 (60%) PB-ASCs compared with 11 of 34 (32.4%) PB-ACs, with no significant difference between the two groups (P=0.11). By contrast, the ID phenotype was less common in PB-ASC (4 of 15; 26.7%) than in PB-AC (22 of 34; 64.7%; P=0.02).
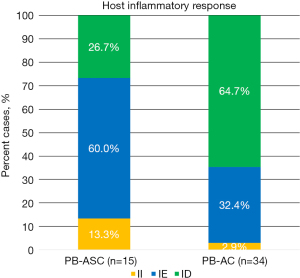
In our series, carcinomas with the II phenotype were rare, and all of them (2 PB-ASCs and 1 PB-AC) were positive for PD-L1. Among the carcinomas with the IE phenotype, PD-L1 expression was observed in 6 of 9 (66.7%) PB-ASCs and 2 of 11 (18.2%) PB-ACs, with no significant difference between the two groups. By contrast, among the carcinomas with the ID phenotype, PD-L1 expression was observed in 2 of 4 (50%) ASCs but not in any of the 22 ACs (0%), and the difference between the two groups was significant (P=0.01).
Discussion
Immunotherapies targeting the PD-1/PD-L1 immune checkpoint have shown encouraging results in solid and hematologic cancers (28-31); however, PD-L1 still has no recognized role in predicting the immunotherapy response of tumors of the pancreaticobiliary system (32-36). We found positive PD-L1 expression in 66.7% of PB-ASCs, which was significantly higher than the frequency of PD-L1 expression in PB-AC (8.8%). Moreover, PD-L1 expression in PB-ASC tended to occur more frequently in the squamous component than in the glandular component, although the difference was not statistically significant. Recently, Zhang et al. demonstrated that higher levels of PD-L1 expression were linked with a shorter overall survival in pancreatic ASC and pancreatic ductal AC (37). This suggests that higher PD-L1 expression on the surface of tumor cells in pancreatic carcinomas enables more effective evasion of antitumor immune responses. Thus, higher PD-L1 expression in PB-ASC might help explain why PB-ASC has a poorer prognosis than PB-AC.
In order to measure PD-L1 expression in tumor tissue, several IHC assays have been developed and received approval from the U.S. Food and Drug Administration. Currently, PD-L1 antibody clones available for clinical studies include 22C3, 28-8, SP263, and SP142. We evaluated PB-ASCs for tumor expression of PD-L1 using the anti-PD-L1 clone E1L3N. The E1L3N clone binds to the cytoplasmic domain of PD-L1, and its binding epitope is not identical to but overlaps considerably with the SP263/SP142 binding site (38). The 22C3 clone is known to have binding profiles in the extracellular domain of PD-L1, and its cellular epitope is different from that of SP263, SP142, and E1L3N (38). Nevertheless, recent studies have shown high concordance of PD-L1 TPS scores between E1L3N and 22C3 (25,39,40). Currently, the E1L3N clone of the PD-L1 antibody is one of the most widely used antibodies approved for research use only, and the aforementioned studies have indicated that E1L3N can be reliably used for screening PD-L1 expression status in a research environment.
Tumor-expressed PD-L1 is a representative biomarker for predicting response to PD-L1/PD-1 inhibitor treatment; however, it is widely accepted that mismatch repair (MMR)-deficient tumors with increased microsatellite instability (MSI) have a higher response rate to immunotherapies targeting the PD-1/PD-L1 axis than MMR-proficient tumors (41). MMR deficiency is common in colorectal, gastric, and endometrial cancers, but it is less common in pancreaticobiliary carcinomas (42-44). In addition to increased MSI, tumor mutation burden (TMB) and tumor-infiltrating immune cells (TICs) have been suggested to positively correlate with the response to PD-L1 blockade therapy (45,46), although many tumors with high TMB also have increased MSI. Hence, in MMR-proficient tumors like pancreaticobiliary carcinomas, assessing biomarkers such as tumor-expressed PD-L1 and TICs may yield useful information. It is noteworthy that in our study, the II and IE phenotypes were more common in PB-ASC (13% and 60%, respectively) than in PB-AC (3% and 32%, respectively). Conversely, the non-inflamed/ID phenotype was more than twice as common in PB-AC than in PB-ASC (65% vs. 27%). This suggests that immune checkpoint therapy might be more effective in patients with PB-ASC than in patients with PB-AC.
PB-ASC is a rare type of malignant exocrine tumor that demonstrates characteristics of both ductal AC and squamous cell carcinoma. Current pathological guidelines for ASC diagnosis of the pancreas and gallbladder require that the squamous component constitute a substantial part of the tumor (≥30% in the pancreas, >25% in the gallbladder) (1), whereas there is no minimum percentage of squamous component required for ASC diagnosis of the extrahepatic bile duct. These cut-off values for PB-ASC diagnosis appear somewhat arbitrary (47-49), and the clinical significance of squamous cell proportionality remains unclear. Voong et al. found that the proportion of squamous differentiation in pancreatic ASC did not influence survival in their series of 38 patients (48). Because ASC has an inferior prognosis compared with AC, some authors propose that any squamous cell component in a pancreatic tumor should be enough to classify the cancer as ASC (47,50). Because the relative importance of the squamous component within ASCs remains unclear, one case of pancreatic carcinoma with glandular and squamous differentiation in which the squamous component was slightly less than 30% (25%, case 9) was included in our study as PB-ASC. Similarly, four cases of pancreatic carcinoma with glandular and squamous differentiation in which the diagnosis was based on FNB or FNA specimens were included as PB-ASCs despite the exact proportion of the squamous component in the tumor not being known.
Several hypotheses for the histopathogenesis of PB-ASC have been proposed, including (I) malignant transformation of metaplastic squamous epithelium induced by chronic inflammation/irritation; (II) transdifferentiation of AC to squamous cell carcinoma; (III) malignant transformation of pluripotent stem cells capable of glandular and squamous differentiation; and (IV) collision of two independent, histologically distinct malignant cell populations (51-54). From a molecular standpoint, two major molecular profiles of pancreatic ductal AC have been identified to date, namely, the classical/non-squamous (or classic/pancreatic) subtype and the squamous (or squamous/basal-like/quasimesenchymal) subtype (55,56). In brief, the squamous subtype usually displays poorly differentiated histomorphologic phenotypes, with <40% of non-gland-forming ducts associated with a squamous and/or adenosquamous carcinoma-like morphology (57-59). Tumor cells of the squamous subtype are usually embedded in a highly cellular stroma enriched with activated/immature cancer-associated fibroblasts and display histological dedifferentiation driven by downregulation of pancreatic endodermal cell-fate-determining genes and activation of the epithelial-to-mesenchymal transition program, which leads to further acquisition of mesenchymal features (55). Thus, ASC of the pancreas is a rare but distinct cancer subtype in which neoplastic cells appear to acquire stemness features through oncogenic dedifferentiation and transdifferentiation.
While knowledge of genetic alterations in gallbladder cancer and extrahepatic cholangiocarcinoma has improved (60,61), molecular data regarding ASC of these anatomical sites is still lacking. The clinical and prognostic impacts of tumor genetics in PB-ASC, particularly ASC of the biliary tract, remains an active area of investigation. Embryological and anatomical studies have shown that the biliary tract represents an incomplete pancreas, and some non-neoplastic and neoplastic biliary diseases (e.g., IgG4-related sclerosing cholangitis, precursor intraepithelial neoplasias, and large-duct-type cholangiocarcinoma) share the pathological features of corresponding pancreatic diseases (62,63). This suggests that information obtained from studies of pancreatic ASC may be applied to analyses of biliary-tract ASC and vice versa, and future therapeutic strategies for pancreatic and biliary ASCs might be developed with a similar or unified approach.
Conclusions
Our investigations demonstrated that PB-ASCs are notably enriched in inflammatory response, and relatively high expression of PD-L1 by PB-ASC tumor cells might be a useful indicator to select patients for immunotherapy. The authors acknowledge that the number of patients in this single-institution pilot study is limited; however, the findings presented here warrant further study with larger samples to develop treatment alternatives for this aggressive subtype of pancreaticobiliary carcinoma.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-9/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-9/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-9/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-9/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was conducted following Institutional Review Board approval, in the Department of Pathology at the University of North Carolina at Chapel Hill (IRB No. 19-1862). Because of the retrospective nature of the study involving only existing data and human biological specimens, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO Classification of Tumours Editorial Board (5th ed). WHO Classification of Tumours of the Digestive System, vol. 1. Lyon: IARC; 2019.
- Boyd CA, Benarroch-Gampel J, Sheffield KM, et al. 415 patients with adenosquamous carcinoma of the pancreas: a population-based analysis of prognosis and survival. J Surg Res 2012;174:12-9. [Crossref] [PubMed]
- Huang Z, Wang J, Zhang R, et al. Pancreatic adenosquamous carcinoma: A population level analysis of epidemiological trends and prognosis. Cancer Med 2023;12:9926-36. [Crossref] [PubMed]
- Xiong Q, Zhang Z, Xu Y, et al. Pancreatic Adenosquamous Carcinoma: A Rare Pathological Subtype of Pancreatic Cancer. J Clin Med 2022;11:7401. [Crossref] [PubMed]
- Roa JC, Tapia O, Cakir A, et al. Squamous cell and adenosquamous carcinomas of the gallbladder: clinicopathological analysis of 34 cases identified in 606 carcinomas. Mod Pathol 2011;24:1069-78. [Crossref] [PubMed]
- Okabayashi T, Kobayashi M, Nishimori I, et al. Adenosquamous carcinoma of the extrahepatic biliary tract: clinicopathological analysis of Japanese cases of this uncommon disease. J Gastroenterol 2005;40:192-9. [Crossref] [PubMed]
- Hong SM, Kim MJ, Jang KT, et al. Adenosquamous carcinoma of extrahepatic bile duct: clinicopathologic study of 12 cases. Int J Clin Exp Pathol 2008;1:147-56. [PubMed]
- Kanagasabapathy S, Subasinghe D, Sivaganesh S, et al. Adenosquamous Carcinoma of the Distal Common Bile Duct: A Case of a Rare Type of Cholangiocarcinoma. Clin Pathol 2022;15:2632010X221099884.
- Qin W, Hu L, Zhang X, et al. The Diverse Function of PD-1/PD-L Pathway Beyond Cancer. Front Immunol 2019;10:2298. [Crossref] [PubMed]
- Chen RY, Zhu Y, Shen YY, et al. The role of PD-1 signaling in health and immune-related diseases. Front Immunol 2023;14:1163633. [Crossref] [PubMed]
- Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res 2020;10:727-42. [PubMed]
- Gao M, Shi J, Xiao X, et al. PD-1 regulation in immune homeostasis and immunotherapy. Cancer Lett 2024;588:216726. [Crossref] [PubMed]
- Wu M, Huang Q, Xie Y, et al. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J Hematol Oncol 2022;15:24. [Crossref] [PubMed]
- Tang Q, Chen Y, Li X, et al. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol 2022;13:964442. [Crossref] [PubMed]
- Wang DR, Wu XL, Sun YL. Therapeutic targets and biomarkers of tumor immunotherapy: response versus non-response. Signal Transduct Target Ther 2022;7:331. [Crossref] [PubMed]
- Wang CC, Huang KT, Chang HC, et al. Comprehensive analysis of PD-L1 in non-small cell lung cancer with emphasis on survival benefit, impact of driver mutation and histological types, and archival tissue. Thorac Cancer 2022;13:38-47. [Crossref] [PubMed]
- Shi X, Wu S, Sun J, et al. PD-L1 expression in lung adenosquamous carcinomas compared with the more common variants of non-small cell lung cancer. Sci Rep 2017;7:46209. [Crossref] [PubMed]
- Li C, Zheng X, Li P, et al. Heterogeneity of tumor immune microenvironment and real-world analysis of immunotherapy efficacy in lung adenosquamous carcinoma. Front Immunol 2022;13:944812. [Crossref] [PubMed]
- Hlaing AM, Furusato B, Udo E, et al. Expression of phosphatase and tensin homolog and programmed cell death ligand 1 in adenosquamous carcinoma of the lung. Biochem Biophys Res Commun 2018;503:2764-9. [Crossref] [PubMed]
- Liu Y, Dong Z, Jiang T, et al. Heterogeneity of PD-L1 Expression Among the Different Histological Components and Metastatic Lymph Nodes in Patients With Resected Lung Adenosquamous Carcinoma. Clin Lung Cancer 2018;19:e421-30. [Crossref] [PubMed]
- Lee SM, Sung CO. PD-L1 expression and surgical outcomes of adenosquamous carcinoma of the pancreas in a single-centre study of 56 lesions. Pancreatology 2021;21:920-7. [Crossref] [PubMed]
- Tanigawa M, Naito Y, Akiba J, et al. PD-L1 expression in pancreatic adenosquamous carcinoma: PD-L1 expression is limited to the squamous component. Pathol Res Pract 2018;214:2069-74. [Crossref] [PubMed]
- Silvestris N, Brunetti O, Pinto R, et al. Immunological mutational signature in adenosquamous cancer of pancreas: an exploratory study of potentially therapeutic targets. Expert Opin Ther Targets 2018;22:453-61. [Crossref] [PubMed]
- Neyaz A, Husain N, Kumari S, et al. Clinical relevance of PD-L1 expression in gallbladder cancer: a potential target for therapy. Histopathology 2018;73:622-33. [Crossref] [PubMed]
- Song L, Zeng L, Yan H, et al. Validation of E1L3N antibody for PD-L1 detection and prediction of pembrolizumab response in non-small-cell lung cancer. Commun Med (Lond) 2022;2:137. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Akhtar M, Rashid S, Al-Bozom IA. PD-L1 immunostaining: what pathologists need to know. Diagn Pathol 2021;16:94. [Crossref] [PubMed]
- Huo G, Liu W, Chen P. Inhibitors of PD-1 in Non-Small Cell Lung Cancer: A Meta-Analysis of Clinical and Molecular Features. Front Immunol 2022;13:875093. [Crossref] [PubMed]
- Yang L, Dong XZ, Xing XX, et al. Efficacy and safety of anti-PD-1/anti-PD-L1 antibody therapy in treatment of advanced gastric cancer or gastroesophageal junction cancer: A meta-analysis. World J Gastrointest Oncol 2020;12:1346-63. [Crossref] [PubMed]
- Drakes ML, Czerlanis CM, Stiff PJ. Immune Checkpoint Blockade in Gynecologic Cancers: State of Affairs. Cancers (Basel) 2020;12:3301. [Crossref] [PubMed]
- Xie W, Medeiros LJ, Li S, et al. PD-1/PD-L1 Pathway: A Therapeutic Target in CD30+ Large Cell Lymphomas. Biomedicines 2022;10:1587. [Crossref] [PubMed]
- Henriksen A, Dyhl-Polk A, Chen I, et al. Checkpoint inhibitors in pancreatic cancer. Cancer Treat Rev 2019;78:17-30. [Crossref] [PubMed]
- Gajiwala S, Torgeson A, Garrido-Laguna I, et al. Combination immunotherapy and radiation therapy strategies for pancreatic cancer-targeting multiple steps in the cancer immunity cycle. J Gastrointest Oncol 2018;9:1014-26. [Crossref] [PubMed]
- Marletta S, Fusco N, Munari E, et al. Atlas of PD-L1 for Pathologists: Indications, Scores, Diagnostic Platforms and Reporting Systems. J Pers Med 2022;12:1073. [Crossref] [PubMed]
- Hu ZI, Lim KH. Evolving Paradigms in the Systemic Treatment of Advanced Gallbladder Cancer: Updates in Year 2022. Cancers (Basel) 2022;14:1249. [Crossref] [PubMed]
- Weinberg BA, Xiu J, Lindberg MR, et al. Molecular profiling of biliary cancers reveals distinct molecular alterations and potential therapeutic targets. J Gastrointest Oncol 2019;10:652-62. [Crossref] [PubMed]
- Zhang Z, Xiong Q, Xu Y, et al. The PD-L1 Expression and Tumor-Infiltrating Immune Cells Predict an Unfavorable Prognosis in Pancreatic Ductal Adenocarcinoma and Adenosquamous Carcinoma. J Clin Med 2023;12:1398. [Crossref] [PubMed]
- Lawson NL, Dix CI, Scorer PW, et al. Mapping the binding sites of antibodies utilized in programmed cell death ligand-1 predictive immunohistochemical assays for use with immuno-oncology therapies. Mod Pathol 2020;33:518-30. [Crossref] [PubMed]
- Munari E, Zamboni G, Lunardi G, et al. PD-L1 expression in non-small cell lung cancer: evaluation of the diagnostic accuracy of a laboratory-developed test using clone E1L3N in comparison with 22C3 and SP263 assays. Hum Pathol 2019;90:54-9. [Crossref] [PubMed]
- Zhang W, Cao Z, Gao C, et al. High concordance of programmed death-ligand 1 expression with immunohistochemistry detection between antibody clones 22C3 and E1L3N in non-small cell lung cancer biopsy samples. Transl Cancer Res 2020;9:5819-28. [Crossref] [PubMed]
- Li H, van der Merwe PA, Sivakumar S. Biomarkers of response to PD-1 pathway blockade. Br J Cancer 2022;126:1663-75. [Crossref] [PubMed]
- Hu ZI, Shia J, Stadler ZK, et al. Evaluating Mismatch Repair Deficiency in Pancreatic Adenocarcinoma: Challenges and Recommendations. Clin Cancer Res 2018;24:1326-36. [Crossref] [PubMed]
- Goeppert B, Roessler S, Renner M, et al. Low frequency of mismatch repair deficiency in gallbladder cancer. Diagn Pathol 2019;14:36. [Crossref] [PubMed]
- Ju JY, Dibbern ME, Mahadevan MS, et al. Mismatch Repair Protein Deficiency/Microsatellite Instability Is Rare in Cholangiocarcinomas and Associated With Distinctive Morphologies. Am J Clin Pathol 2020;153:598-604. [Crossref] [PubMed]
- Daud AI, Loo K, Pauli ML, et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest 2016;126:3447-52. [Crossref] [PubMed]
- Valero C, Lee M, Hoen D, et al. Response Rates to Anti-PD-1 Immunotherapy in Microsatellite-Stable Solid Tumors With 10 or More Mutations per Megabase. JAMA Oncol 2021;7:739-43. [Crossref] [PubMed]
- Borazanci E, Millis SZ, Korn R, et al. Adenosquamous carcinoma of the pancreas: Molecular characterization of 23 patients along with a literature review. World J Gastrointest Oncol 2015;7:132-40. [Crossref] [PubMed]
- Voong KR, Davison J, Pawlik TM, et al. Resected pancreatic adenosquamous carcinoma: clinicopathologic review and evaluation of adjuvant chemotherapy and radiation in 38 patients. Hum Pathol 2010;41:113-22. [Crossref] [PubMed]
- Kardon DE, Thompson LD, Przygodzki RM, et al. Adenosquamous carcinoma of the pancreas: a clinicopathologic series of 25 cases. Mod Pathol 2001;14:443-51. [Crossref] [PubMed]
- Murakami Y, Yokoyama T, Yokoyama Y, et al. Adenosquamous carcinoma of the pancreas: preoperative diagnosis and molecular alterations. J Gastroenterol 2003;38:1171-5. [Crossref] [PubMed]
- Moslim MA, Lefton MD, Ross EA, et al. Clinical and Histological Basis of Adenosquamous Carcinoma of the Pancreas: A 30-year Experience. J Surg Res 2021;259:350-6. [Crossref] [PubMed]
- Boecker W, Tiemann K, Boecker J, et al. Cellular organization and histogenesis of adenosquamous carcinoma of the pancreas: evidence supporting the squamous metaplasia concept. Histochem Cell Biol 2020;154:97-105. [Crossref] [PubMed]
- Qin BD, Jiao XD, Yuan LY, et al. Adenosquamous carcinoma of the bile duct: a population-based study. Cancer Manag Res 2018;10:439-46. [Crossref] [PubMed]
- Gulwani HV, Gupta S, Kaur S. Squamous Cell and Adenosquamous Carcinoma of Gall Bladder: a Clinicopathological Study of 8 Cases Isolated in 94 Cancers. Indian J Surg Oncol 2017;8:560-6. [Crossref] [PubMed]
- Gutiérrez ML, Muñoz-Bellvís L, Orfao A. Genomic Heterogeneity of Pancreatic Ductal Adenocarcinoma and Its Clinical Impact. Cancers (Basel) 2021;13:4451. [Crossref] [PubMed]
- Aguirre AJ, Nowak JA, Camarda ND, et al. Real-time Genomic Characterization of Advanced Pancreatic Cancer to Enable Precision Medicine. Cancer Discov 2018;8:1096-111. [Crossref] [PubMed]
- Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47-52. [Crossref] [PubMed]
- Hayashi A, Fan J, Chen R, et al. A unifying paradigm for transcriptional heterogeneity and squamous features in pancreatic ductal adenocarcinoma. Nat Cancer 2020;1:59-74. [Crossref] [PubMed]
- N Kalimuthu S. Morphological classification of pancreatic ductal adenocarcinoma that predicts molecular subtypes and correlates with clinical outcome. Gut 2020;69:317-28. [Crossref] [PubMed]
- Kuipers H, de Bitter TJJ, de Boer MT, et al. Gallbladder Cancer: Current Insights in Genetic Alterations and Their Possible Therapeutic Implications. Cancers (Basel) 2021;13:5257. [Crossref] [PubMed]
- Montal R, Sia D, Montironi C, et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J Hepatol 2020;73:315-27. [Crossref] [PubMed]
- Nakanuma Y, Sudo Y. Biliary tumors with pancreatic counterparts. Semin Diagn Pathol 2017;34:167-75. [Crossref] [PubMed]
- Zaccari P, Cardinale V, Severi C, et al. Common features between neoplastic and preneoplastic lesions of the biliary tract and the pancreas. World J Gastroenterol 2019;25:4343-59. [Crossref] [PubMed]


