Preoperative chemoradiation and IOERT for unresectable or borderline resectable pancreas cancer
Introduction
In locally unresectable pancreas cancer, the use of external beam irradiation (EBRT) with concurrent chemotherapy results in a doubling of median survival when compared with surgical bypass or stents alone and an increase in 2 year overall survival (OS) from 0-5% to 10-20% (1-4). However, five-year OS is rare, and local control is low even with doses of 60-70 Gy in 1.8-2 Gy fractions over 7-8 weeks (3-4).
The combination of EBRT and intraoperative electrons has resulted in an improvement in local control in IOERT series from Massachusetts General Hospital (MGH), Mayo Clinic and other institutions (5-10). This did not, however, translate into major improvements in either median or two-year survival.
In an attempt to improve patient selection and survival, investigators from Mayo Clinic Cancer Center - Rochester (MCCC-R) delivered the concurrent chemoradiation component of treatment before restaging, exploration and IOERT in a series of 27 patients (11). Although two-year survival appeared to be improved with the altered sequence of preoperative treatment followed by IOERT, this was presumably due to altered patient selection, as the relative incidence of liver plus peritoneal relapse did not change (14 of 27 patients at risk or 52%).
Our institution has used the sequence of preoperative chemoradiation (preop CRT) followed by restaging, surgical exploration with resection and IOERT, as indicated, for select patients with locally advanced pancreas cancer. This retrospective review was performed to evaluate survival, relapse patterns, tolerance and prognostic factors.
Methods and materials
Between January 2002 and December 2010, 48 patients with locally advanced unresectable or borderline resectable pancreatic ductal adenocarcinoma (ACA) received preop CRT prior to an attempt at resection and IOERT. Resection was not attempted in 17 of the 48 patients for the following reasons: disease progression at restaging in 12 (9 patients, preoperative imaging; 3 patients, peritoneal seeding at laparoscopy prior to surgical exploration and attempted resection); patient declined surgery, 2; medically inoperable, 3. A retrospective review of the 31 patients who underwent attempted resection is presented here.
Patient and disease factors
Patient factors were evaluated with regard to sex, age and performance status (Table 1). There were 13 females and 18 males with median age of 64 (range, 41-85). Performance status (PS) was 0 or 1 in all patients (PS 0 =18, PS 1 =13).
Full Table
Information that was collected with regard to potential disease prognostic factors included: resection status prior to preop CRT, site of lesion, grade and CA 19-9 level (Table 1). Site of the primary lesion was in the pancreatic head in 20 patients and body in 11 patients. The tumor grade was moderately differentiated ductal ACA in 5 patients, poorly differentiated in 18 patients, and not specified in 8 patients. Resection status prior to preop CRT was categorized by the surgeon, radiologist, and radiation oncologist as locally unresectable in 20 patients and borderline resectable in 11. Prior to 2007, definitions of borderline resectable were not standardized, but the local strategy was to consider tumors involving but without encasement of the celiac or superior mesenteric artery and amenable to possible venous resection/reconstruction. In more recent years, definitions of borderline resectable disease became standardized as described in the publication of Varadhachary et al. (12).
Treatment information
Treatment factors were collected with regard to irradiation, surgery and chemotherapy (Table 1). This included type of concurrent chemotherapy [gemcitabine vs. 5-fluourouracil (5-FU)], dose and method of EBRT, degree of surgical resection (R0, R1, R2, unresectable), dose of IOERT, and use of maintenance chemotherapy. The concurrent chemotherapy was 5-FU-based in 11 patients [protracted venous infusion (PVI), 6; capecitabine, 2; 5-FU/Oxaliplatin, 3] or gemcitabine-based in 18 patients (weekly single-agent gemcitabine, 12; gemcitabine doublet, 2). The type of concurrent chemotherapy was unknown in 2 patients who received the preop CRT at another institution.
EBRT was delivered at our institution for 27 patients and at outside institutions for 4 patients. Treatments were designed using high energy photons and either 3-D conformal, multi-field techniques (27 patients) or intensity modulated radiation therapy (4 patients). Treatment fields included both the primary tumor and nodal areas at risk. Techniques used in our institution have been described in detail in prior publications and will only be summarized (1,6,9,11). The EBRT dose was 45-50.4 Gy in 25-28 fractions (Fx) of 1.8 Gy in 27 patients. A boost field was carried to 54-56 Gy in 28-30 Fx in 2 patients. The EBRT dose was <45 Gy in 2 patients because of intolerance to the treatment (39.6 Gy/22 Fx; 43.2 Gy/24 Fx).
Surgical resection was feasible in 17 of 31 patients after preop CRT (R0 in 11 patients; R1 in 5; R2 in 1) and the lesion was unresectable in 14 patients. Whipple resection was performed in 9 patients with primary lesions in the head of pancreas, and the other 8 patients had a distal pancreatectomy with splenectomy for primary lesions in the body of the pancreas. A vascular sleeve resection and reconstruction was necessary in 2 patients (superior mesenteric vein - 1; left renal vein - 1).
IOERT was given as a component of treatment in 28 of 31 patients. IOERT was delivered with a mobile electron accelerator (Mobetron®; Sunnyvale, Ca). The IOERT dose was based on both the extent of resection and the dose of preop EBRT: R0 resection, 12.5 Gy; R1, median 12.5 Gy (range, 10-15 Gy); R2, 15 Gy; unresectable 17.5 Gy (n=2) or 20 Gy (n=12). IOERT energy was based on the depth of the tumor bed or unresected tumor, and IOERT applicator size included the tumor bed or unresected tumor with a 1-cm margin (e.g., 4 cm tumor/tumor bed =6 cm applicator).
Systemic maintenance chemotherapy was preferred in all patients but given in only 16 of 31 (unknown in 3). Maintenance chemotherapy was gemcitabine-based in all patients who received additional therapy. Neoadjuvant chemotherapy was given prior to preop CRT in 7 patients consisting of several cycles of gemcitabine plus nab-paclitaxel.
Outcomes
Outcomes evaluated include survival [overall (OS) and disease-free (DFS)], disease relapse [local failure in the EBRT field (LF), central failure in the IOERT field (CF) and distant metastases (DM)] and treatment tolerance (during preop CRT, the peri-operative period, and the 30-day post-operative period). OS and DFS were calculated with the Kaplan-Meier method (13). Differences between Kaplan-Meier curves were calculated with the log-rank test (univariate analyses). Both survival and time to relapse were calculated from initiation of treatment.
Results
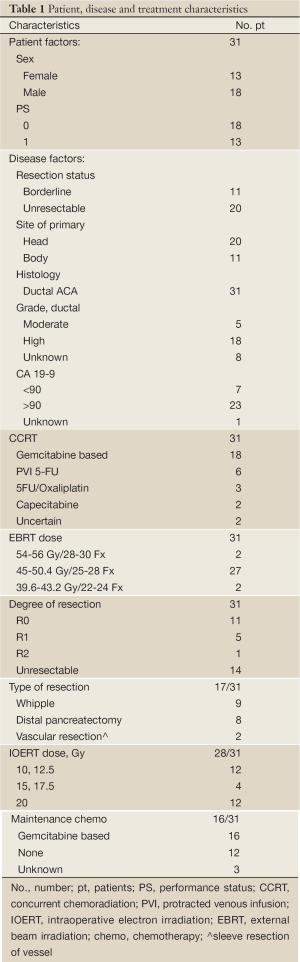
Patient status was evaluated at time of analysis with median follow-up of 19 months for all patients and 31 months for survivors. Median survival for the entire group of 31 patients was 19 months with 2-year OS of 31% and 3-year OS of 16% (Table 2, Figure 1A). Three patients were alive with no evidence of disease (31, 33, 79 months), 2 alive with disease relapse (22, 21 months). Twenty-three patients were dead of disease (median 17 months, range, 4-75 months); two had died with no evidence of disease at 4 months (massive CVA) and 7 months (perforated viscous due to stent) and 1 had died with an uncertain status at 14 months.
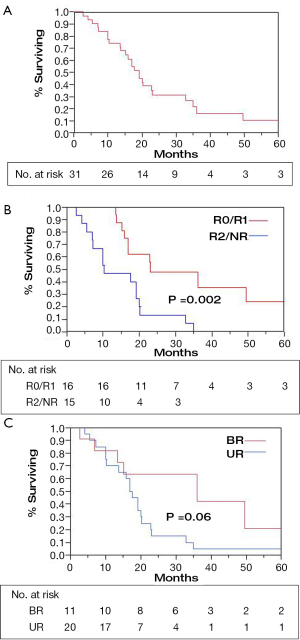
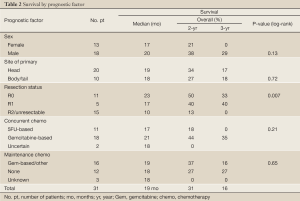
Resection status was the only significant predictor for survival (Table 2). When an R0 or R1 resection was achieved vs. R2 resection or unresectable disease, 2-year OS was 48% vs. 13% and 3-year OS was 36% vs. 0% (Figure 1B; P=0.002 log-rank). An OS advantage approached statistical significance for patients considered borderline resectable vs. unresectable in pre-treatment evaluation (Figure 1C; 2-year OS 63% vs. 15%, P=0.06 log rank). Other factors such as sex, site of the primary lesion, initial CA 19-9 level, change in CA 19-9 level with therapy, type of concurrent chemotherapy during EBRT, or maintenance chemotherapy (yes/no) were not prognostic for improved OS (Table 2). The DFS at 1 and 2 years was 64% and 20%, respectively, with a median of 13 months. No factors, including extent of surgical resection, predicted for improved DFS.
Full Table
Disease relapse
Sites of relapse were evaluated in the total group of 31 patients (Table 3). LF/CF was documented in 5 of 31 patients (16%). The incidence of LF/CF in patients who underwent resection (1/17; 6%) was lower compared to patients with unresectable disease (4/14; 29%), but this difference was not statistically significant. DM was documented in 24/31 patients (77%). Sites of metastatic failure included the liver (11 patients), peritoneum (10 patients), or lung/pleura/mediastinum (10 patients). Abdominal relapse in the liver or peritoneal cavity was documented in 22 of 31 patients (71%); the incidence did not differ by resection status, as noted in Table 3.

Full Table
Treatment tolerance
Preop CRT was generally well tolerated. The EBRT dose was attenuated to <45 Gy/25 Fx in 2/31 patients (6%; Table 1) because of gastrointestinal intolerance (39.6 Gy/22 Fx; 43.2 Gy/24 Fx). Peri-operative morbidity and mortality also were analyzed. Grade 3 or 4 peri-operative morbidity was seen in 7/31 patients (23%). Re-operation was required in 4 patients [3 of 4 within 30 days: pancreatic leak/wound infection (1 patient), wound dehiscence (1 patient), wound dehiscence and small bowel obstruction (1 patient); 1 of 4 patients at post-operative day 49 with a gastro-jejunostomy leak]. An additional 3 patients required re-admission for ileus, dehydration or abscess within 30 days but were managed conservatively. There were no peri-operative deaths.
Discussion
EBRT plus chemotherapy
Long-term disease-control and survival are infrequent for patients with locally unresectable pancreas cancer treated with EBRT or CRT alone. CRT results in a doubling of median survival when compared with surgical bypass or stents alone (3-6 months vs. 9-13 months) and an increase in 2-year OS from 0-5% to 10-20% (1-4). Five-year survivors are rare, however, and local control is not common. Even with EBRT doses of 60-70 Gy in 1.8 to 2 Gy fractions, local failure was documented in at least two-thirds of the patients in a series from Thomas Jefferson University (3,4).
EBRT plus IORT
The combination of EBRT plus intraoperative electrons for patients with locally unresectable pancreas cancer resulted in an improvement in local control in IOERT series from MGH, Mayo and other institutions (5-10). This did not, however, translate into major improvements in either median or two-year survival.
In the most recent update of results from Massachusetts General Hospital, 150 patients with locally unresectable pancreas ductal ACA received IOERT as a component of treatment from 1978 to 2001 in conjunction with EBRT and 5-FU based chemotherapy (14). Long-term survival was seen in 8 patients and 5 were alive at or beyond the 5-year interval. Actuarial 1-, 2-, 3- and 5-year OS for the 150 patients was 54%, 15%, 7% and 4% respectively and median survival was 13 months. Survival was significantly related to the diameter of the IOERT treatment applicator (surrogate for tumor size). In the 26 patients treated with a 5 or 6 cm applicator, 2- and 3-year OS were 27% and 17%; 0/11 patients treated with a 9 cm diameter applicator survived beyond 18 months and those treated with a 7 or 8 cm applicator had intermediate survival (P<0.05).
In the initial Mayo Clinic Cancer Center—Rochester (MCCC-R) IORT series of patients with locally unresectable pancreas ACA, IOERT usually preceded EBRT (6). When results were compared with EBRT ± 5-FU (no IOERT), local control at one year was 82% for EBRT plus IORT ± 5-FU versus 48% for EBRT ± 5-FU; at two years it was 66% versus 20%, respectively (P=0.0005). The improvement in local control did not, however, translate into a difference in either median or two-year OS (13.4 months median OS with IOERT versus 12.6 months without; 12% versus 16.5% 2-year OS). The lack of survival improvement was related to a high incidence of abdominal failure in both groups of patients (20 of 37 IOERT patients, or 54% developed liver or peritoneal metastases versus 68 of 122 or 56% in non-IOERT patients).
Pre-operative therapy
In an attempt to improve patient selection and survival, investigators from MCCC-R delivered the EBRT plus chemo before restaging and exploration (11). In 27 patients with locally unresectable pancreas ACA who received IOERT after preoperative CRT, local control was achieved in 21 patients (78% with actuarial rates of 86% and 68% at 1 and 2 years, respectively). Median survival was 14.9 months with this sequence and 2 and 5 year OS were respectively 27% and 7%. These findings were compared with results in 56 patients who had IOERT before receiving the high dose external component at Mayo or elsewhere (median OS 10.5 months, 2 year OS 6%, P=0.001). In an earlier analysis of 37 patients treated solely at Mayo with the latter sequence, median and 2 year survival were respectively 13.6 months and 12%. Although 2-year OS appeared to improve with the altered sequence of preoperative CRT followed by IOERT, this was likely due to altered patient selection as the rate of liver plus peritoneal relapse did not change (14 of 27 patients at risk, 52%).
Aristu et al. from Spain reviewed 47 patients with locally unresectable ACA treated with EBRT in combination with a variety of chemotherapy regimens including 5FU, cisplatin, gemcitabine, docetaxel, or paclitaxel (15). After neoadjuvant therapy, 12 patients were felt to be potentially resectable, and surgical resection was achieved in 9 patients. Surgical exploration was undertaken in an additional 21 unresectable patients, and 6 received IOERT (median dose 16 Gy, range, 10-29 Gy). Median survival was 23 months and 10 months for resected or unresectable patients, respectively. OS at 3 years was 48% when resection was achieved, but none of the unresected patients were alive at 2 years.
Additional series have evaluated the benefits of preop CRT without IOERT for patients initially deemed to be surgically unresectable, realizing that this definition may differ markedly by institution and also by surgeon within a given institution. Snady et al. reported on a patient cohort treated with split course XRT concurrent with 5FU and cisplatin (16). Of the initial 68 patients, 30 patients (44%) underwent surgical exploration, and, of these, resection was successfully achieved in 20 patients. Survival was significantly longer in patients where resection was achieved compared to no resection (median, 2-year, and 3-year overall survival: 31 vs. 21 months; 61% vs. 34%; 32% vs. 13%). Interestingly, patients who underwent resection after preop CRT were found to have longer OS compared to a cohort of patients who had been deemed resectable at diagnosis and underwent primary resection (median, 2-year, and 3-year OS: 14 months, 31%, 14%.) Ammori and colleagues at the University of Michigan reviewed their experience of 67 patients with locally unresectable disease treated with gemcitabine and preop RT (17). After initial pre CRT, 17 patients underwent exploration and resection was achieved in 9 patients with median OS 17.9 months. Median survival in unresected patients was 11.9 months.
Borderline resectable disease
A consensus definition of borderline resectable disease has been a relatively recent development (18). This group of patients would appear to be ideal candidates for preoperative treatment strategies. Katz and colleagues at MD Anderson Cancer Center have reported the largest experience thus far (19). Their prospective database was retrospectively reviewed for patients who were not candidates for primary surgery and treated with preoperative chemoradiation strategies. Among this cohort of patients who received neoadjuvant therapy were 84 patients with anatomically borderline resectable disease. After preop CRT, 32 patients underwent surgical resection (31 R0/1 R1). Median survival in resected patients was 40 months compared to 15 months for unresected patients.
A small experience of only 13 patients with radiographic borderline resectable disease was reported by Brown et al. from Fox Chase Cancer Center (20). Treatment regimens included 50.4 Gy EBRT with either gemcitabine or 5FU-based concurrent chemotherapy followed by a median of 3 cycles of full dose chemotherapy, which was typically gemcitabine-based. Surgery was then performed at a median of 8 months from diagnosis. Of the 13 patients treated with neoadjuvant therapy and surgery, 8 patients were alive without disease at 24 months follow-up.
Recently, Barugola and colleagues from Italy compared the outcomes of 41 patients diagnosed with unresectable or borderline resectable pancreatic cancer and who were treated either with neoadjuvant chemotherapy alone or with chemoradiation followed by successful surgical resection to 362 resectable patients treated with upfront surgery (21). There was no increase in operative morbidity or mortality. Compared to patients receiving neoadjuvant chemotherapy alone, neoadjuvant chemoradiation significantly improved both the rate of pathologic complete response (0% vs. 12.5%; P=0.03%) and the rate of R0 resection (35% vs. 96%; P<0.001). No statistically significant difference in OS survival was observed between the patients who received neoadjuvant therapy group and those treated with upfront surgical resection (median survival 35 vs. 27 months; P=0.74). Therefore, despite imaging suggestive of greater local disease, neoadjuvant chemoradiation could be successfully combined with surgical resection to achieve equivalent results as primary surgery.
Current series
For select patients with borderline resectable or locally unresectable pancreas cancer, we have used only the sequence of preoperative CRT followed by restaging, surgical exploration with resection/IOERT, as indicated. Of the 48 patients who received preop CRT, 31 proceeded to surgical exploration, and an R0/R1 resection was achieved in 16 patients. IOERT was included in the treatment for 28 of the 31 resected patients. Extent of surgical resection was the most important factor impacting survival with a 3-year OS of 36% after R0/R1 resection (Figure 1B). Despite the use of IOERT in the setting of unresectable disease, no patients were alive at 3 years after R2 resection or with unresectable disease. These findings are consistent with other studies in the literature described above. In addition, abdominal relapse in the liver or peritoneal cavity has been documented in 22 of 31 patients (71%) in the current series. These relapse rates parallel findings in multiple prior analyses from MCCC-R that include both resectable (22) and unresectable pancreas cancer patients (6,8,11).
The current series is limited by its retrospective nature and limited patient numbers. Although dedicated pancreatic CT and MRI imaging was used throughout the study time period, the imaging technology has improved and the sequence protocols have evolved significantly over the past decade. The definitions of locally unresectable vs. borderline resectable disease were not standardized based on strict radiographic criterion during much of the early time period studied. However, decisions regarding the use of neoadjuvant therapy were made in a multidisciplinary setting. Our findings are consistent with and add to the limited data available for this patient population.
Conclusions
The current series confirms that long-term survival and disease control are achievable in select patients with borderline resectable or locally unresectable pancreas cancer (1-12,14,19,23-32). Therefore, continued evaluation of curative-intent combined modality therapy is warranted in this high-risk population of patients. Although some investigators have deleted irradiation as a component of treatment for patients with locally unresectable cancers, the phase III trial from the Eastern Cooperative Oncology Group (E4201) demonstrated an advantage in OS with involved field EBRT plus concurrent gemcitabine compared to gemcitabine alone (P=0.04, 2-sided log rank) for such patients (33).
Survival appears to be better in patients with resection after full-dose preoperative CRT in the current MCCC-A series and is a sequencing strategy that will be continued in our institution. Preop CRT has also been a preferred strategy for this group of patients in other institutions, in an attempt to improve resection rates (19,23-32,34-36). Preop CRT is the preferred treatment at MD Anderson Cancer Center (MDACC) even for patients with resectable cancers, based on imaging criterion (23-27,35,36).
Additional strategies are needed, to improve both resectability rates after preoperative CRT and disease control (local, distant). Improvements in imaging continue to allow better selection of patients in whom gross total resection alone or plus IOERT may be feasible after preoperative treatment for initially unresectable or borderline resectable pancreas cancers.
However, significant improvements in long-term survival for borderline resectable and unresectable pancreatic cancer patients will not occur until abdominal and systemic relapse rates can be markedly reduced with more effective systemic therapy. In patients with resected pancreas cancer, adjuvant gemcitabine has been shown to improve both DFS and OS when compared to surgery alone in phase III trials (37,38). A recent phase III trial has demonstrated that combination chemotherapy with the FOLFIRINOX regimen improved survival compared to gemcitabine alone in the setting of metastatic disease but has been tested only in a limited fashion in the adjuvant/neoadjuvant setting. The incidence of abdominal relapse may be decreased either by utilizing more aggressive or new regimens of systemic therapy (39) and/or regional therapy (intrahepatic, intraperitoneal) and evaluating altered sequencing of treatment with regard to systemic and local components of treatment. Targeted therapies (e.g., epidermal growth factor receptor (EGFR) inhibitors, vascular endothelial growth factor (VEGF) inhibitors) and pancreas cancer vaccines are also being evaluated in an attempt to improve systemic disease control (40). Gemcitabine plus nab-paclitaxel has shown substantial anti-tumor activity in a phase I/II trial in metastatic pancreas cancer patients with an overall response rate of 48% (39); gemcitabine alone has comparative response rates of 5-15%. A >20% decrease in CA 19-9 values was found in 92% of patients. Data in additional patients accrued to the trial was consistent with initial results and is the basis for a phase III trial.
Delivery of several cycles of gemcitabine-based systemic therapy prior to concurrent CRT is being evaluated in our and other institutions (MDACC, UCSF, other) in an attempt to achieve better systemic control of micro-metastases prior to consolidating the local-regional component of treatment (41,42). As more effective concurrent CRT and systemic therapies are developed, both disease control and survival outcomes should improve in patients with locally unresectable and borderline resectable pancreas ACA.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Foo ML, Gunderson LL, Urrutia R. Pancreatic cancer. In: Gunderson LL, Tepper JE. eds. Clinical Radiation Oncology. New York: Churchill Livingstone/Harcourt Health Sciences Co., 2000: 686-706.
- Abrams RA, Choo J. Pancreatic Cancer. In: Gunderson LL, Tepper JE. eds. Clinical Radiation Oncology, 2 Edition. Philadelphia: Churchill Livingstone/Elsevier, 2007:1061-82.
- Whittington R, Solin L, Mohiuddin M, et al. Multimodality therapy of localized unresectable pancreatic adenocarcinoma. Cancer 1984;54:1991-8. [PubMed]
- Mohiuddin M, Cantor RJ, Biermann W, et al. Combined modality treatment of localized unresectable adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys 1988;14:79-84. [PubMed]
- Shipley WU, Wood WC, Tepper JE, et al. Intraoperative electron beam irradiation for patients with unresectable pancreatic carcinoma. Ann Surg 1984;200:289-96. [PubMed]
- Roldan GE, Gunderson LL, Nagorney DM, et al. External beam versus intraoperative and external beam irradiation for locally advanced pancreatic cancer. Cancer 1988;61:1110-6. [PubMed]
- Tepper JE, Shipley WU, Warshaw AL, et al. The role of misonidazole combined with intraoperative radiation therapy in the treatment of pancreatic carcinoma. J Clin Oncol 1987;5:579-84. [PubMed]
- Gunderson LL, Martin JK, Kvols LK, et al. Intraoperative and external beam irradiation +/- 5-FU for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 1987;13:319-29. [PubMed]
- Mohiuddin M, Regine WF, Stevens J, et al. Combined intraoperative radiation and perioperative chemotherapy for unresectable cancers of the pancreas. J Clin Oncol 1995;13:2764-8. [PubMed]
- Termuhlen P, Evans D, Willett CG. IORT in pancreatic carcinoma. In: Gunderson LL, Willett CG, Harrison LB, et al. eds. Intraoperative Irradiation: Techniques and Results. Totowa NJ: Humana Press, 1999:201-22.
- Garton GR, Gunderson LL, Nagorney DM, et al. High-dose preoperative external beam and intraoperative irradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 1993;27:1153-7. [PubMed]
- Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13:1035-46. [PubMed]
- Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457-81.
- Willett CG, Del Castillo CF, Shih HA, et al. Long-term results of intraoperative electron beam irradiation (IOERT) for patients with unresectable pancreatic cancer. Ann Surg 2005;241:295-9. [PubMed]
- Aristu J, Cañón R, Pardo F, et al. Surgical resection after preoperative chemoradiotherapy benefits selected patients with unresectable pancreatic cancer. Am J Clin Oncol 2003;26:30-6. [PubMed]
- Snady H, Bruckner H, Cooperman A, et al. Survival advantage of combined chemoradiotherapy compared with resection as the initial treatment of patients with regional pancreatic carcinoma. An outcomes trial. Cancer 2000;89:314-27. [PubMed]
- Ammori JB, Colletti LM, Zalupski MM, et al. Surgical resection following radiation therapy with concurrent gemcitabine in patients with previously unresectable adenocarcinoma of the pancreas. J Gastrointest Surg 2003;7:766-72. [PubMed]
- Abrams RA, Lowy AM, O’Reilly EM, et al. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol 2009;16:1751-6. [PubMed]
- Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008;206:833-46; discussion 846-8. [PubMed]
- Brown KM, Siripurapu V, Davidson M, et al. Chemoradiation followed by chemotherapy before resection for borderline pancreatic adenocarcinoma. Am J Surg 2008;195:318-21. [PubMed]
- Barugola G, Partelli S, Crippa S, et al. Outcomes after resection of locally advanced or borderline resectable pancreatic cancer after neoadjuvant therapy. Am J Surg 2012;203:132-9. [PubMed]
- Foo ML, Gunderson LL, Nagorney DM, et al. Patterns of failure in grossly resected pancreatic ductal adenocarcinoma treated with adjuvant irradiation +/- 5 fluorouracil. Int J Radiat Oncol Biol Phys 1993;26:483-9. [PubMed]
- Hoffman JP, Weese JL, Solin LJ, et al. A single institutional experience with preoperative chemoradiotherapy for stage I-III pancreatic adenocarcinoma. Am Surg 1993;59:772-80; discussion 780-1. [PubMed]
- Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol 2001;8:123-32. [PubMed]
- White RR, Hurwitz HI, Morse MA, et al. Neoadjuvant chemoradiation for localized adenocarcinoma of the pancreas. Ann Surg Oncol 2001;8:758-65. [PubMed]
- Wayne JD, Abdalla EK, Wolff RA, et al. Localized adenocarcinoma of the pancreas: the rationale for preoperative chemoradiation. Oncologist 2002;7:34-45. [PubMed]
- Pisters PW, Wolff RA, Crane CH, et al. Combined-modality treatment for operable pancreatic adenocarcinoma. Oncology (Williston Park) 2005;19:393-404, 409; discussion 409-10, 412-6.
- Jessup JM, Steele G Jr, Mayer RJ, et al. Neoadjuvant therapy for unresectable pancreatic adenocarcinoma. Arch Surg 1993;128:559-64. [PubMed]
- Bajetta E, Di Bartolomeo M, Stani SC, et al. Chemoradiotherapy as preoperative treatment in locally advanced unresectable pancreatic cancer patients: results of a feasibility study. Int J Radiat Oncol Biol Phys 1999;45:285-9. [PubMed]
- Wanebo HJ, Glicksman AS, Vezeridis MP, et al. Preoperative chemotherapy, radiotherapy, and surgical resection of locally advanced pancreatic cancer. Arch Surg 2000;135:81-7; discussion 88. [PubMed]
- Mehta VK, Fisher G, Ford JA, et al. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. J Gastrointest Surg 2001;5:27-35. [PubMed]
- Magnin V, Moutardier V, Giovannini MH, et al. Neoadjuvant preoperative chemoradiation in patients with pancreatic cancer. Int J Radiat Oncol Biol Phys 2003;55:1300-4. [PubMed]
- Loehrer PJ Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol 2011;29:4105-12. [PubMed]
- Mornex F, Girard N, Scoazec JY, et al. Feasibility of preoperative combined radiation therapy and chemotherapy with 5-fluorouracil and cisplatin in potentially resectable pancreatic adenocarcinoma: The French SFRO-FFCD 97-04 Phase II trial. Int J Radiat Oncol Biol Phys 2006;65:1471-8. [PubMed]
- Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3487-95. [PubMed]
- Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3496-502. [PubMed]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [PubMed]
- Neuhaus P, Riess H, Post S, et al. CONKO-001: final results of the randomized, prospective, multicenter phase III trial of adjuvant chemotherapy with gemcitabine versus observation in patients with resected pancreas cancer. ASCO Proceedings. J Clin Oncol 2008;26:214.
- Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 2011;29:4548-54. [PubMed]
- Philip PA, Mooney M, Jaffe D, et al. Consensus report of the national cancer institute clinical trials planning meeting on pancreas cancer treatment. J Clin Oncol 2009;27:5660-9. [PubMed]
- Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer 2007;110:47-55. [PubMed]
- Ko AH, Quivey JM, Venook AP, et al. A phase II study of fixed-dose rate gemcitabine plus low-dose cisplatin followed by consolidative chemoradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2007;68:809-16. [PubMed]


