
Inhibition of GABAergic neurons in the paraventricular nucleus of the hypothalamus precipitates visceral pain induced by pancreatic cancer in mice
Highlight box
Key findings
• Targeting GABAergic neurons alleviated visceral pain induced by pancreatic cancer.
What is known and what is new?
• The paraventricular nucleus of the hypothalamus (PVN) has previously been implicated in the modulation of visceral pain.
• PVN regulates the visceral pain associated with pancreatic cancer in mice by inhibiting GABAergic neurons.
What is the implication, and what should change now?
• Targeting GABAergic neurons in PVN may emerge as a novel approach for treating visceral pain in pancreatic cancer.
Introduction
Pain is an unpleasant sensory and emotional affective experience associated with, or similar to, actual or potential tissue damage (1). Pain represents a subjective encounter that serves as an adaptive and protective mechanism; however, it can also exert detrimental effects on physical functioning, mental well-being, and social interactions. Chronic pain, defined as enduring or recurring for more than 3 months, has been recognized as a distinct disorder. Chronic visceral pain caused by pancreatic cancer is a prevalent form of pain encountered in clinical practice. Abdominal pain is highly prevalent among patients with pancreatic cancer (2), with advanced pain occurring in up to 90% of cases (3,4). However, the underlying mechanism of visceral pain in pancreatic cancer remains elusive, leading to a lack of specific and efficacious treatment options for this condition. Consequently, there is an urgent need to investigate the neural basis of visceral pain in pancreatic cancer.
Previously, we and others have found that the paraventricular nucleus of the hypothalamus (PVN) is involved in regulating visceral pain (5-7). We demonstrated that intra-PVN administration of either the small-conductance Ca2+-activated K+ channel 2 inhibitor apamin or protein kinase A (PKA) activator 8-Br-cAMP effectively attenuates PVN neuronal activity, leading to a reduction in visceral pain in irritable bowel syndrome in mice. Besides, recent findings indicate that PVN exhibits innervation of pancreatic β-cells in the endocrine region (8), suggesting its significance as a pivotal central nervous system nucleus involved in pancreas innervation. Therefore, we are attempting to investigate whether the PVN is involved in regulating visceral pain in pancreatic cancer and its specific neural mechanisms.
Excitation/inhibition imbalance underlies numerous pathological conditions (5,9). Given our previous findings that inhibiting the excitability of PVN neurons can alleviate visceral pain (5), we hypothesized that GABAergic neurons—the primary inhibitory neurons—are involved in regulating visceral pain. In this study, we established a pancreatic cancer mouse model, in which we demonstrated persistent visceral pain. Utilizing the visceral pain model, we investigated the functional alterations of GABAergic neurons. In vitro electrophysiological results showed that the firing frequency of GABAergic neurons in the PVN was decreased. Specific destruction of GABAergic neurons in the PVN exacerbated visceral pain induced by pancreatic cancer. Chemogenetics activation of GABAergic neurons in the PVN alleviated visceral pain induced by pancreatic cancer.
Our findings suggest that the suppression of PVN GABAergic inhibitory neurons plays a pivotal role in the development of visceral pain induced by pancreatic cancer in mice. Activation of GABAergic inhibitory neurons through chemogenetics effectively alleviates visceral pain, thereby highlighting their potential as novel intervention agents for managing this type of pain. We present this article in accordance with the ARRIVE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-50/rc).
Methods
Animals
Male C57B/6N mice, aged 6 weeks, were procured from the Experimental Animal Center of Xuzhou Medical University (Xuzhou, China). The mice were housed under controlled environmental conditions, including a consistent temperature (22±2 ℃) and 50%±5% relative humidity, a 12-h light-dark cycle. Food and water were available ad libitum. This study was approved by the Animal Care and Use Committee of Shanghai Ninth People’s Hospital (No. SH9H-2023-A223-SB), in compliance with the national guidelines for the care and use of animals. A protocol was prepared before the study without registration. Male mice were randomly and blindly separated into each group according to random number table method.
Mouse model of pancreatic cancer visceral pain
The mouse model of pancreatic cancer visceral pain was conducted according to previously described methods (10). C57BL/6 male mice were intrapancreatically injected with mPAKPC-luc cells (GemPharmatech, Nanjing, China) suspended in 100 µL mixed medium [Matrigel: phosphate-buffered saline (PBS) =1:1] using a sterile insulin needle, establishing an orthotopic tumor model. The control group underwent the same procedure as the model group, except for the administration of mPAKPC-luc cells. When the mice in the experimental process meet the following welfare criteria, euthanasia would be conducted based on animal welfare standards, using excessive inhalation of 95% CO2 to induce death: (I) persistent diarrhea; (II) sluggishness (inability to eat or drink); (III) hunched back and lying on their side; (IV) reduced activity and symptoms of muscle atrophy; (V) difficulty breathing; (VI) progressive decrease in body temperature; (VII) paralysis and convulsions; (VIII) continuous bleeding; (IX) inability for animals to move normally due to large tumors or other reasons; and (X) inability for animals to move normally due to severe ascites or increased abdominal circumference.
Behavioral analysis
Visceral pain was assessed by abdominal mechanical hyperalgesia test and hunch score. The observations were conducted by two independent observers who were blinded to the experimental status of the mouse in all instances.
Abdominal mechanical hyperalgesia test
Behavioral analyses were performed as described previously with some modifications (11,12). A total of 0.16 g Von Frey fiber (IITC Inc. Life Science, Woodland Hills, CA, USA) was vertically stimulated to the left upper abdomen for about 2 seconds, with a stimulation interval of 5 minutes, and this was repeated 10 times. Positive reactions were defined by the presence of the following behaviors: lifting, scratching, licking the abdomen, and moving or jumping immediately. Response frequency (%) = (Positive response/10 trials) × 100.
Hunch score
The hunch score was employed as a means of assessing spontaneous visceral pain and was examined following previously described protocols with some modifications (13). The scoring criteria for hunch behavior were as follows: 0—absence of round-back posture, displaying exploratory behavior, and normal hair with a glossy sheen; 1—mild round-back posture, characterized by exploratory behavior and normal hair with a glossy sheen; 2—severe round-back posture, marked by a slight reduction in exploratory behavior, mild piloerection, and current episodes of abdominal muscle contractions; 3—severe round-back posture, marked by significantly reduced exploratory behavior, moderate piloerection, and recurrent episodes of abdominal muscle contractions; 4—severe round-back posture, characterized by minimal or no exploratory behavior, complete piloerection throughout the entire body, and lack of movement in the head region. The hunch score was computed by averaging over a 300-second duration.
Electrophysiological experiment
After implanting tumors in mice for 12 days, we stimulated the abdominal pancreas of the mice with a 0.16 g filament for approximately 2 seconds, with a 5-minute interval between each stimulation, repeated 10 times. Half an hour later, mice were deeply anesthetized and transcardially perfused with 95% O2 and 5% CO2 oxygenated ice-cold cutting solution comprising (in mM): 93 N-methyl-D-glucamine (NMDG), 93 HCl, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 25 D-glucose, 20 hydroxyethyl piperazine ethanesulfonic acid (HEPES), 5 Na-ascorbate, 2 thiourea, 3 Na-pyruvate, 10 MgSO4, and 0.5 CaCl2, pH 7.35 with NMDG or HCl. The cortical slices containing PVN (300 µm) were sectioned using a Leica VT1200s vibratome (Leica, Wetzlar, Germany), incubated in cutting solution, and then maintained at 25 ℃ in oxygenated artificial cerebrospinal fluid (ACSF; in mM: 124 NaCl, 3 KCl, 2 CaCl2 1.3 MgCl2, 25 NaHCO3, 1.25 NaH2PO4, and 10 glucose) for a duration of one hour prior to recordings.
The recording pipettes were filled with a solution containing the following concentrations (in mM): 135 K-gluconate, 5 KCl, 0.5 CaCl2, 10 HEPES, 2 Mg-adenosine triphosphate (ATP), 0.1 guanosine triphosphate (GTP), and 5 ethylene glycol tetraacetic acid (EGTA). The osmolarity was adjusted to 300 milliosmole (mOsm) and the pH was maintained at 7.3 by using KOH. The liquid-junction potential was not taken into account when correcting the membrane potentials. The frequency of AP was defined as the number of occurrences within a specific time interval. The elicitation of action potentials (APs) was achieved by applying current injections lasting 400 ms at 10 different intensities (20, 40, 60, 80, 100, 120, 140, 160, 180 and 200 pA), with a 30-s trial interval. Resting membrane potentials (RMP) were measured within 1 minute of break-in to the whole-cell configuration. The threshold was defined as the membrane potential at which the slope of the phase plot exceeded 15 mV/ms.
The signals were obtained utilizing a MultiClamp 700B amplifier (Molecular Devices, San Jose, CA, USA). The number of neurons and mice are indicated in the respective figure legends.
Brain stereotactic injection
Mice were anesthetized (isoflurane, induction concentration 5%, maintenance concentration 2%) and quickly fixed to a stereoscope. After the head hair had been shaved, the skin was cleaned with 70% alcohol, and an incision was made in the scalp, and then the periosteum tissue on the skull was wiped away with hydrogen peroxide. To inject the virus, a small hole was drilled into the skull at the target location, and 100 nL was injected with a microinjector. After the injection, the microinjector was left in place for 10 minutes, then slowly pulled out. After the scalp was sutured, the mice were returned to the cage and given plenty of water and food. The coordinates of the PVN region were targeted using Paxinos and Franklin’s Atlas as the reference: (anteroposterior) 0.94 mm, (mediolateral) ±0.20 mm, and (dorsoventral) 5.10 mm from bregma.
Ablation of glutamatergic neurons
For targeted ablation of PVN GABAergic neurons, rAAV-Dlx5/6-taCasp3-T2A-TEVp-WPREs-pA or rAAV-Dlx5/6-EGFP-WPREs-pA (100 nL; BrainVTA, Wuhan, China) was bilaterally injected into the PVN of anesthetized model mice after 9 days of tumor implantation. Behavioral assessments were performed on days 14, 17, and 20 following viral injection. Subsequently, the distribution of viral fluorescent protein expression in the PVN was examined using fluorescence microscopy.
Chemogenetics inhibition of GABAergic neurons
For targeted activation of GABAergic neurons in the PVN, mice were injected with AAV-Dlx5/6-hM3Dq-mCherry or AAV-Dlx5/6-mCherry vectors into the PVN 9 days after tumor implantation. Behavioral assessments were conducted on days 14, 17, and 20 post virus injection. Prior to behavioral assessments, both groups of mice received an intraperitoneal injection of Clozapine N-oxide (CNO) at a concentration of 0.33 mg/mL (0.2 mL/20 g) administered 40 minutes before testing. Following the behavioral tests, viral fluorescent protein expression in the PVN was visualized using fluorescence microscopy.
Statistical analysis
All data were presented as mean ± standard deviation (SD). All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). Independent samples Student’s t-test was used to analyze statistical differences between two groups. Comparisons between multiple groups were performed by 2-way analysis of variance (2-way ANOVA) followed by post hoc Bonferroni. Wilcoxon rank sum test was used to compare hunch score. A P value less than 0.05 was considered statistically significant.
Results
PVN GABAergic neuronal activity was inhibited in mice with pancreatic cancer visceral pain
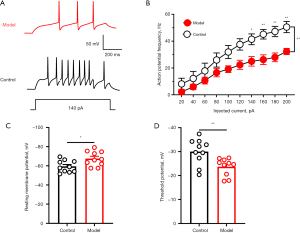
We examined the electrophysiological properties of glutamatergic neurons in the PVN using in vitro brain slices. Representative samples of APs in PVN were obtained from a control mouse and a model mouse (Figure 1A). The AP frequency was decreased in orthotropic pancreatic cancer mouse model (Figure 1B). The RMP was decreased in orthotropic pancreatic cancer mouse model (Figure 1C). The threshold potential was increased in the orthotropic pancreatic cancer mouse model (Figure 1D). These results suggested that pancreatic cancer-induced pain caused inactivity of PVN GABAergic neurons.
Specific destruction of PVN GABAergic neurons exacerbated visceral pain induced by pancreatic cancer
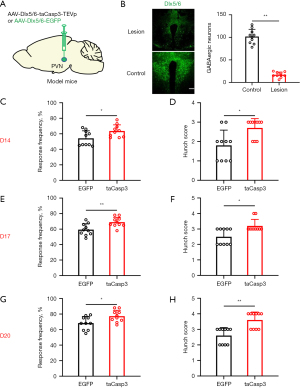
Subsequently, we selectively ablated GABAergic neurons in the PVN using taCasp3 to investigate their involvement in visceral pain associated with pancreatic cancer. The administration of taCasp3 resulted in a progressive reduction of GABAergic neurons within the PVN, leading to an average loss of 83.2% of GABAergic cells 20 days post virus injection (Figure 2A,2B). Visceral pain was evaluated through abdominal mechanical hyperalgesia testing and hunch score assessments on days 14 (Figure 2C,2D), 17 (Figure 2E,2F), and 20 (Figure 2G,2H) following viral injection. Notably, significant differences were observed in both abdominal mechanical hyperalgesia responses and hunch scores at these time points after pancreatic virus injection in mice. These behavioral findings suggest that targeted elimination of PVN GABAergic neurons partially exacerbates visceral pain associated with pancreatic cancer.
Chemogenetics activation of PVN GABAergic neurons alleviated visceral pain induced by pancreatic cancer
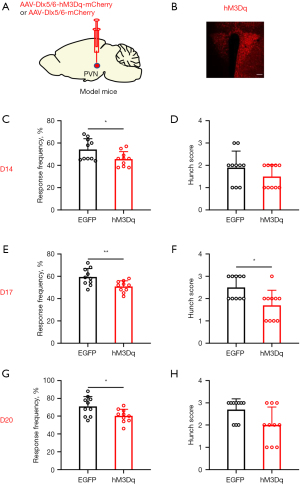
Conversely, we employed chemogenetics to selectively activate GABAergic neurons in order to investigate their involvement in visceral pain associated with pancreatic cancer. The accuracy of virus injection location was confirmed through fluorescence imaging (Figure 3A,3B). Visceral pain was evaluated using the abdominal mechanical hyperalgesia test and hunch score on days 14, 17, and 20 following virus injection. Significant differences were observed in both abdominal mechanical hyperalgesia and hunch score on days 14 (Figure 3C,3D), 17 (Figure 3E,3F), and 20 (Figure 3G,3H) after pancreatic injection of the virus in mice. Behavioral findings demonstrated that specific inhibition of PVN GABAergic neurons alleviated visceral pain induced by pancreatic cancer.
Discussion
In this study, in order to reveal the role of PVN GABAergic neurons in pancreatic cancer visceral pain, we made a pancreatic cancer visceral pain mouse model. In vitro electrophysiological results showed that the firing frequency of GABAergic neurons in the PVN was decreased. Specific destruction of GABAergic neurons in the PVN exacerbated visceral pain induced by pancreatic cancer. Chemogenetics activation of GABAergic neurons in the PVN alleviated visceral pain induced by pancreatic cancer. Our findings suggested that the PVN was involved in the development of pancreatic visceral pain, and specific regulation of GABAergic neurons in the PVN can alleviate pancreatic visceral pain, providing new insights for the discovery of effective targets for the treatment of pancreatic visceral pain.
Our previous studies, as well as those conducted by others, have shown that PVN can regulate visceral pain through sensitization of corticotropin-releasing hormone (CRH) neurons (5-7). Therefore, reducing PVN neuron excitability appears to be a viable approach for alleviating visceral pain. In line with this, our earlier research demonstrated that activation of SK2 channels reduced neuronal excitability and relieved visceral pain in irritable bowel syndrome (5). Similarly, Song et al. (14) found that disinhibition of GABAergic neurons projecting to the PVN contributed to the excitation of CRH neurons and mediated visceral hypersensitivity. Chemogenetic activation of these PVN-projecting neurons also alleviated visceral hypersensitivity. Consistent with these findings, our results indicate that GABAergic neuron excitability is decreased in pancreatic cancer-induced visceral pain and specific ablation of GABAergic neurons further exacerbates this pain. Conversely, chemogenetics activation of GABAergic neurons significantly relieves pancreatic cancer-induced visceral pain. Therefore, we suggest that in mice with visceral pain, GABA released by GABAergic neurons is reduced, which leads to increased excitability of CRH neurons and mediates pancreatic cancer visceral pain. By activating GABA neurons, the release of GABA is increased to alleviate pancreatic cancer visceral pain. These results further support the feasibility of reducing PVN neuron excitability for alleviating visceral pain.
There are also some studies that seem to contradict this, suggesting that activating the PVN can alleviate visceral pain. For example, Su et al. (15) found a protective effect of microinjection of glutamate into the hypothalamic PVN on chronic visceral hypersensitivity in rats. We believe that these seemingly contradictory results further highlight the important role of PVN in visceral pain. The PVN is a heterogeneous nucleus containing various types of neurons (16), which determines its complex functionality. Arginine vasopressin (AVP)-positive neurons in the PVN project to the solitary tract nucleus; therefore, activating AVP-positive neurons can protect intestinal mucosa through the vagus nerve and relieve visceral pain (14). Additionally, oxytocin-positive neurons exist in the PVN and have significant analgesic effects (17-19); thus, activating oxytocin-positive neurons can also alleviate visceral pain (20,21). Therefore, we believe that future research should focus on studying the specific roles of different types of PVN neurons in visceral pain and identifying protective versus harmful neurons for more targeted modulation and better relief from visceral pain.
Conclusions
Our research suggests that the inhibition of GABAergic neurons in PVN plays a crucial role in the development of visceral pain caused by pancreatic cancer in mice. By activating these inhibitory neurons using chemogenetics, we effectively alleviated visceral pain, highlighting their potential as innovative intervention agents for managing pancreatic cancer visceral pain.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-50/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-50/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-50/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-50/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All experimental procedures were approved by the Animal Care and Use Committee of Shanghai Ninth People’s Hospital (No. SH9H-2023-A223-SB), in compliance with the national guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- IASP. Pain terms and definitions. 2022. Available online: https://www.iasp-pain.org/resources/terminology/#pain
- Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694-705. [Crossref] [PubMed]
- van Geenen RC, Keyzer-Dekker CM, van Tienhoven G, et al. Pain management of patients with unresectable peripancreatic carcinoma. World J Surg 2002;26:715-20. [Crossref] [PubMed]
- Lindsay TH, Jonas BM, Sevcik MA, et al. Pancreatic cancer pain and its correlation with changes in tumor vasculature, macrophage infiltration, neuronal innervation, body weight and disease progression. Pain 2005;119:233-46. [Crossref] [PubMed]
- Ji NN, Du L, Wang Y, et al. Small-Conductance Ca(2+)-Activated K(+) Channels 2 in the Hypothalamic Paraventricular Nucleus Precipitates Visceral Hypersensitivity Induced by Neonatal Colorectal Distension in Rats. Front Pharmacol 2021;11:605618. [Crossref] [PubMed]
- Huang ST, Wu K, Guo MM, et al. Glutamatergic and GABAergic anteroventral BNST projections to PVN CRH neurons regulate maternal separation-induced visceral pain. Neuropsychopharmacology 2023;48:1778-88. [Crossref] [PubMed]
- Ji NN, Kang J, Hua R, et al. Involvement of dopamine system in the regulation of the brain corticotropin-releasing hormone in paraventricular nucleus in a rat model of chronic visceral pain. Neurol Res 2018;40:650-7. [Crossref] [PubMed]
- Rosario W, Singh I, Wautlet A, et al. The Brain-to-Pancreatic Islet Neuronal Map Reveals Differential Glucose Regulation From Distinct Hypothalamic Regions. Diabetes 2016;65:2711-23. [Crossref] [PubMed]
- Li S, Huang H, Wei X, et al. The recycling of AMPA receptors/GABAa receptors is related to neuronal excitation/inhibition imbalance and may be regulated by KIF5A. Ann Transl Med 2022;10:1103. [Crossref] [PubMed]
- Wang M, Wu M, Liu X, et al. Pyroptosis Remodeling Tumor Microenvironment to Enhance Pancreatic Cancer Immunotherapy Driven by Membrane Anchoring Photosensitizer. Adv Sci (Weinh) 2022;9:e2202914. [Crossref] [PubMed]
- Selvaraj D, Hirth M, Gandla J, et al. A mouse model for pain and neuroplastic changes associated with pancreatic ductal adenocarcinoma. Pain 2017;158:1609-21. [Crossref] [PubMed]
- Yu D, Zhu J, Zhu M, et al. Inhibition of Mast Cell Degranulation Relieves Visceral Hypersensitivity Induced by Pancreatic Carcinoma in Mice. J Mol Neurosci 2019;69:235-45. [Crossref] [PubMed]
- Sevcik MA, Jonas BM, Lindsay TH, et al. Endogenous opioids inhibit early-stage pancreatic pain in a mouse model of pancreatic cancer. Gastroenterology 2006;131:900-10. [Crossref] [PubMed]
- Song Y, Meng QX, Wu K, et al. Disinhibition of PVN-projecting GABAergic neurons in AV region in BNST participates in visceral hypersensitivity in rats. Psychoneuroendocrinology 2020;117:104690. [Crossref] [PubMed]
- Su Z, Miao B, Xu MQ, et al. Protective effect of microinjection of glutamate into hypothalamus paraventricular nucleus on chronic visceral hypersensitivity in rats. Brain Res 2020;1747:147048. [Crossref] [PubMed]
- Liu Y, Li A, Bair-Marshall C, et al. Oxytocin promotes prefrontal population activity via the PVN-PFC pathway to regulate pain. Neuron 2023;111:1795-1811.e7. [Crossref] [PubMed]
- Chen S, Xu H, Dong S, et al. Morpho-Electric Properties and Diversity of Oxytocin Neurons in Paraventricular Nucleus of Hypothalamus in Female and Male Mice. J Neurosci 2022;42:2885-904. [Crossref] [PubMed]
- Li YJ, Du WJ, Liu R, et al. Paraventricular nucleus-central amygdala oxytocinergic projection modulates pain-related anxiety-like behaviors in mice. CNS Neurosci Ther 2023;29:3493-506. [Crossref] [PubMed]
- Li J, Liu H, Guo F, et al. Increased GABAergic projections in the paraventricular nucleus regulate colonic hypersensitivity via oxytocin in a rat model of irritable bowel syndrome. Neuroreport 2023;34:108-15. [Crossref] [PubMed]
- Gamal-Eltrabily M, Espinosa de Los Monteros-Zúñiga A, Manzano-García A, et al. The Rostral Agranular Insular Cortex, a New Site of Oxytocin to Induce Antinociception. J Neurosci 2020;40:5669-80. [Crossref] [PubMed]
- Tsushima H, Zhang Y, Muratsubaki T, et al. Oxytocin antagonist induced visceral pain and corticotropin-releasing hormone neuronal activation in the central nucleus of the amygdala during colorectal distention in mice. Neurosci Res 2021;168:41-53. [Crossref] [PubMed]


