
Two autopsied gastric cancer cases of rare drug-induced pneumonia associated with nivolumab plus S-1 and oxaliplatin: a case report
Highlight box
Key findings
• We encountered two fatal cases of drug-induced pneumonia caused by nivolumab plus S-1 and oxaliplatin for gastric cancer and performed an autopsy of these two cases.
What is known and what is new?
• Fatal pneumonia is rare in the clinical trial of this regimen.
• This regimen can cause diffuse alveolar damage with limited response to corticosteroids; even computed tomography images show organizing pneumonia pattern.
What is the implication, and what should change now?
• Patients undergoing this regimen should be closely followed up with imaging, evaluation of symptom including oxygen saturation and serological marker analysis, such as C-reactive protein, Krebs von den Lungen-6, and lactate dehydrogenase.
Introduction
Cancer chemotherapy accounts for 23–51% of drug-induced pneumonia cases, and they are sometimes fatal (1). Especially, prevalence of immune checkpoint inhibitors (ICI)-related interstitial lung disease (ILD) is reported to be 14.6–19.0% in non-small cell lung cancer (2). Risk factors are smoking history, pre-existing lung disease, previous drug-induced pneumonia, Eastern Cooperative Oncology Group performance status (ECOG PS) ≥2 at cancer diagnosis, and advanced stage of the underlying cancer. Asian ethnicity is also said to be a risk factor. Physical examination, history taking, and differential diagnosis from other pneumonia are essential for diagnosis. Treatment is discontinuation of the drug and sometimes immunosuppressive therapy (1).
Nivolumab plus S-1 and oxaliplatin therapy is now used as the first-line treatment for human epidermal growth factor receptor 2 (HER2)-negative unresectable or recurrent gastric cancer according to the result of the clinical trial ATTRACTION-4, a randomized, multicenter, double-blind, placebo-controlled, phase 3 trial (3,4). This trial showed significantly improved progression-free survival and an acceptable safety profile.
In the nivolumab plus chemotherapy group (n=359) of this trial, grades 1–2 pneumonia occurred in 3 patients (<1%), and grade 3 occurred in 4 patients (1%). Grades 1–2 ILD was observed in 5 patients (1%), and grade 3 occurred in 3 patients (<1%). No grade 4 or 5 pneumonia or ILD was observed (4). Chinese multicenter retrospective study also revealed no patient of grade 3 or greater pneumonitis from 138 patients (5). This safety profile implies that this regimen has good tolerability for lung injuries, though these studies include limited and selected patients and can miss rare adverse events. Here we report two autopsied cases of fatal drug-induced pneumonia and describe clinical and pathological findings. This is the first report of critical pneumonia from this regimen. We present this article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-511/rc).
Case presentation
Case 1
The patient was a 76-year-old man without a history of smoking or dust exposure. He was diagnosed with gastric cancer and underwent distal gastrectomy in May 2013 [poorly differentiated > moderately differentiated adenocarcinoma, pT1bN3M0, stage IIIA; Union for International Cancer Control (UICC), seventh edition]. The patient received adjuvant chemotherapy for 4 years and 7 months with S-1 and 3 courses of cisplatin. In January 2022, abdominal ultrasound sonography (US) and computed tomography (CT) showed peritoneal dissemination nodules, and the patient was diagnosed with recurrence of gastric cancer.
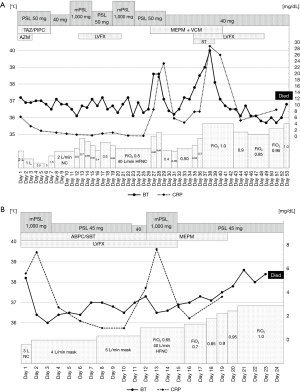
From February, nivolumab plus S-1 and oxaliplatin combination therapy was administered (performance status 1). The fifth dose included 360 mg/body of nivolumab, 80 mg/day of S-1, and 150 mg/body (100 mg/m2) of oxaliplatin, which was administered to the patient in May. Elevated C-reactive protein (CRP) (5.61 mg/dL) and Krebs von den Lungen-6 (KL-6) (498 U/mL) levels from baseline (1.38 mg/dL and 196 U/mL, respectively) without any respiratory symptoms were observed at that time. Retrospectively, 1 month before admission, CT showed slight subpleural dorsal ground-glass opacities in both lungs and pleural effusion in the right lung (Figure 1A).
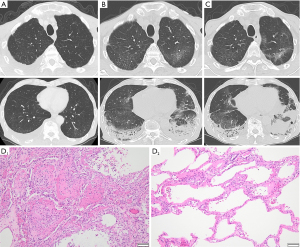
On day 11 of the fifth dose, the patient had fever and dyspnea and further elevation of the CRP (6.26 mg/dL) and KL-6 (774 U/mL) levels (Table 1). CT showed subpleural dorsal consolidation, ground-glass opacities in both lungs, and a small amount of bilateral pleural effusion (Figure 1B). He required 2 L/min of oxygen via nasal cannula and was admitted on the same day. The nasal swab of severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) antigen test was negative. The urinary Streptococcus pneumoniae antigen test was positive, and we diagnosed the patient with drug-induced pneumonia with an organizing pneumonia (OP) pattern and bacterial pneumonia. Antibiotics, tazobactam-piperacillin and azithromycin, and 50 mg/day (1 mg/kg) of prednisolone were administered. The sputum culture result did not show pathogenic bacteria, and blood beta-D-glucan and cytomegalovirus (CMV) antigenemia findings were negative. His oxygenation temporarily improved, and prednisolone was reduced to 40 mg/day on day 8 of admission.
Table 1
| Parameters | Case 1 | Case 2 |
|---|---|---|
| WBC (×103/μL) | 7.5 | 7.6 |
| Baso (%) | 0.0 | 0.0 |
| Eo (%) | 0.0 | 4.0 |
| Stab (%) | 91.0 | 0.0 |
| Seg (%) | 4.0 | 78.0 |
| Ly (%) | 4.0 | 11.0 |
| Mo (%) | 5.0 | 7.0 |
| RBC (×106/μL) | 2.93 | 2.29 |
| Hb (/dL) | 11.6 | 8.7 |
| Ht (%) | 32.7 | 26.1 |
| Plt (×105/μL) | 255 | 313 |
| LDH (U/L) | 221 | 291 |
| CRP (mg/dL) | 6.26 | 5.60 |
| KL-6 (U/mL) | 774 | 538 |
In both cases, the data show elevation of the neutrophil count, CRP, and KL-6 levels from baseline. Case 2 had slight elevation of the LDH level. Urinary Streptococcus pneumoniae antigen was positive in case 1, and Legionella antigen was negative in both cases. Sputum and blood culture result did not show any pathogen, and nasal swab of SARS-CoV2 antigen test was negative in both cases. WBC, white blood cell; Baso, basophil; Eo, eosinophil; Stab, stab neutrophil; Seg, segmented neutrophil; Ly, lymphocyte; Mo, monocyte; RBC, red blood cell; Hb, hemoglobin; Ht, hematocrit; Plt, platelet; CRP, C-reactive protein; KL-6, Krebs von den Lungen-6; LDH, lactate dehydrogenase; SARS-CoV2, severe acute respiratory syndrome coronavirus 2.
On day 12, he developed sudden hypoxia with slight exacerbation of ground-glass opacities on CT (Figure 1C) and needed high-flow nasal therapy [fraction of inspired oxygen (FiO2) 0.5, 40 L/min]. We initiated steroid pulse therapy [3 days of methylprednisolone (1,000 mg/day)] and reduced the dose to 50 mg/day of prednisolone with levofloxiacin. The patient showed a slight improvement in oxygenation for a few days and exacerbation again. We started steroid pulse therapy again and gained tentative improvement, but he developed septic shock due to an unknown pathogen. Although meropenem and vancomycin improved sepsis, hypoxia and fever recurred on day 35. We administered levofloxacin and sulfamethoxazole-trimethoprim for a few days against Pneumocystis jirovecii pneumonia, but the beta-D-glucan result was negative. CT showed exacerbation of interstitial opacity and pneumomediastinum. It was difficult to increase the dose of corticosteroids because of side effects, so we continued supportive care. On day 39, decreased platelet count (5×103/µL), prolonged prothrombin time-international normalized ratio (PT-INR) (1.32), and elevation of fibrinogen/fibrin degradation products (FDP) (51.3 µg/mL) were observed and suggested disseminated intravascular coagulation (DIC). He died on day 53 of admission and underwent an autopsy.
In the pathological examination, we found severe fibrosis with heterogeneous distribution in both lungs. Histologically, we observed diffuse alveolar damage (DAD) and acute fibrinous and organizing pneumonia (AFOP), characterized by intraalveolar fibrinous exudation and “fibrin balls” formation (Figure 1D, D1). The dorsal side of the lower lobes showed the organizing phase of DAD, and some parts of his lungs showed the exudative phase (Figure 1D, D2). This intermingled pattern of acute and subacute lung injury indicated repetitive exacerbation. Although some residual cancer cells were microscopically seen in the subserosal layer of the stomach, macroscopic peritoneal disseminated nodules were not found, which were previously detected by US and CT before the chemotherapy.
Case 2
The patient was a 74-year-old man with no history of smoking or exposure to dust. He was diagnosed with gastric cancer (poorly differentiated adenocarcinoma, sT4aNxM1, stage IVB; UICC, eighth edition) with peritoneal dissemination and underwent gastrojejunal bypass surgery in March 2022.
From May 2022, nivolumab plus S-1 and oxaliplatin therapy was administered (performance status 1). The fifth dose included of 360 mg/body nivolumab, 80 mg/body of S-1, and 190 mg/body (130 mg/m2) of oxaliplatin, and he experienced coughing on day 10 of this dose. From day 20 with this dose, dyspnea was exacerbated, and he was admitted on day 22. CRP, KL-6, lactate dehydrogenase (LDH) level increased to 5.6 mg/dL, 538 U/mL and 291 U/L, respectively (Table 1). He had a fever and required 3 L/min of oxygen via nasal cannula. CT showed bilateral subpleural dorsal consolidation and ground-glass opacities, mainly in the lower lobes (Figure 2A). We diagnosed him with drug-induced pneumonia with the OP pattern and initiated steroid pulse therapy [3 days of methylprednisolone (1,000 mg/day)]. Bacterial pneumonia could not be excluded, and we also prescribed ampicillin-sulbactam and levofloxacin. The nasal swab of SARS-CoV2 antigen test was negative. The sputum culture result did not show pathogenic bacteria, and blood beta-D-glucan and CMV antigenemia results were negative. Prednisolone was reduced to 45 mg/day from day 4 and 40 mg/day from day 11 of admission.
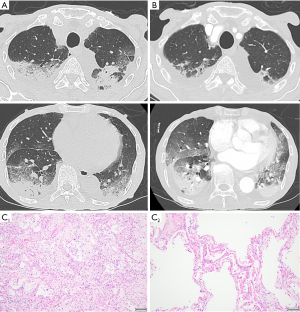
On day 12, hypoxia rapidly exacerbated, and he required high-flow nasal therapy (FiO2 0.6, 40 L/min). CT on this day revealed slight improvement in the upper lobe and impairment of the lower lobe opacities (Figure 2B). We started steroid pulse therapy again and switched the antibiotic to meropenem. The dose of prednisolone was reduced to 45 mg/day from day 15. Hypoxia did not improve, and he died on day 24 of admission.
In the pathological examination, we found DAD in both lungs, including the organizing (Figure 2C, C1) and exudative phases (Figure 2C, C2), indicating repetitive lung injury. We endoscopically observed a tumor mass in the stomach before treatment, but it disappeared macroscopically in the autopsy. We only microscopically found some cancer cells in the muscularis propria and subserosa of the stomach wall. We also found small myocardial and renal infarctions, implying hypercoagulability, as seen in DIC.
The clinical course of both cases is shown in Figure 3.
All procedures performed in this study were in accordance with the ethical standards of the institutional committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients’ families for publication of this case report and accompanying images. Copies of the written consent are archived at Japan Red Cross Society Himeji Hospital, Hyogo, Japan, and are available for review by the editorial office of this journal.
Discussion
The ATTRACTION-4 trial showed that S-1, oxaliplatin, and nivolumab therapy caused no grade 4 or 5 lung injury (4). However, each medication, S-1 (6), oxaliplatin (7), and nivolumab (8), has been reported to cause fatal pneumonia. A small cohort study of S-1 plus oxaliplatin or nab-paclitaxel did not show pulmonary toxicity, and this implies the adverse effect came from nivolumab. Among patients with melanoma, post-marketing surveillance revealed a 5.0% incidence of ILD, including 1.8% of grade 3 or worse, despite this global study showing only 2.3% of ILD and no patients with grade 3 or worse (9). The current report shows a discrepancy between clinical trial data and daily practice, which sometimes matters, as in our cases. According to a systematic review, anti-PD-1/PD-L1 receptor antibody like nivolumab more often cause pneumonia than anti-CTLA-4 receptor antibody (10). As to gastric cancer, gastrointestinal and endocrine immune-related adverse events (irAE) are often reported with anti-PD-1 receptor antibody (11).
National Comprehensive Cancer Network (NCCN) Guidelines recommends discontinuation of immunotherapy and methylprednisolone/prednisolone 1–2 mg/kg/day in grade 2 or worse pneumonitis. It also recommends infectious workups such as viral pathogens, sputum culture, or bronchoscopy with bronchoalveolar lavage. It recommends considering empiric broad-spectrum antibiotics if an infection has not yet been fully excluded. If no improvement is observed after 48 hours, it suggests considering additional immunosuppression with infliximab, intravenous immunoglobulin, or mycophenolate mofetil (12). Infliximab and cyclophosphamide have been reported to be potentially effective, but there is no firm evidence of immunosuppressors (13,14).
According to their time course, we diagnosed them with drug-induced pneumonia/ILD. They can also be described as acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) secondary to drug administration.
We identified some common features between these 2 patients. Before treatment, they did not have interstitial lung abnormalities, which is a risk factor for ILD induced by ICI and acute exacerbation (15-17). Pneumonia developed after the fifth dose of the regimen. CT showed a pattern similar to that of OP, but the pathological pattern was DAD. ICI-related ILD, especially the OP pattern, has good steroid responsiveness (18,19), but our patients showed only a limited and tentative response. There was a slight exacerbation of the CT findings despite the sudden deterioration of hypoxia. High-resolution CT sometimes misses the pathological early phase of DAD, although it is useful for detecting it (20). In the terminal phase, we observed DIC-like coagulation disorder in both cases. Severe pulmonary injury, such as ALI/ARDS and acute exacerbation of idiopathic pulmonary fibrosis is reported to cause DIC (21,22). Anti-PD-1/PD-L1 antibodies can also cause DIC as toxicity via producing inflammatory cytokines (23). Our cases had the both components and led to intractable inflammation. irAEs are related to a better objective tumor response (24,25), and our cases also indicate this relationship, because the anticancer effect was macroscopically complete in these cases. As to gastric cancer, peritoneal metastasis and poorer differentiation are prognostic factors of poor survival (26). The response to the treatment of these cases is promising for patients with poorer prognosis with former treatments, as long as they are under adequate control of adverse effects.
In addition, case 1 had slight opacities on CT 1 month before admission and elevated CRP and KL-6 levels 10 days before admission. Case 2 had respiratory symptoms 10 days before admission. These findings indicate that regular evaluation of imaging and serous markers, besides symptoms and oxygen saturation, can help in the early detection of pneumonia. It is also helpful to increase patient awareness of respiratory symptom. We can avoid ICI when the patient has risk factors. In addition to pneumonia, the trial revealed 11.4% of endocrine event (4), and every 2−3 weeks monitoring is recommended (12). Gastrointestinal and hepatic adverse event are also often seen (35.9% and 23.1% respectively) in this regimen. To further verify the role of nivolumab plus S-1 and oxaliplatin, it is necessary to accumulate clinical cases, because ATRACTION-4 trial included selected and limited number of patients.
Some limitations remain in this report. We did not perform a drug-induced lymphocyte stimulation test (DLST) to diagnose drug-induced pneumonia or to identify a responsible drug, though some reports that DLST is not reliable for drug-induced pneumonia (27). The effects of immunosuppressors on our cases are not evaluable for these cases, because we did not administer them because of the lack of adequate evidence. The reason why these patients with few risk factors for drug-induced pneumonia came to fatal consequence is not clear. Retrospectively, we can see signs of pneumonia and we could achieve better results from earlier discontinuation of the treatment. Surgical or transbronchial lung biopsy can be useful to diagnose ILD, but it is difficult for the patients with acute respiratory failure (28). Bronchoalveolar lavage could suggest some informative findings in the early phase of slight respiratory failure.
Conclusions
This is the first report on fatal pneumonia due to ATTRACTION-4 regimen without potential risk factors, and it emphasize the importance of detecting early indication. We should carefully monitor patients receiving this regimen from the perspective of drug-induced pneumonia. Regular examination of lung imaging, oxygen saturation, assessment of serous markers (CRP, KL-6, and LDH), and history taking of symptoms can help stop chemotherapy and early treatment.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-511/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-511/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-511/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients’ families for publication of this case report and accompanying images. Copies of the written consent are archived at Japan Red Cross Society Himeji Hospital, Hyogo, Japan, and are available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Conte P, Ascierto PA, Patelli G, et al. Drug-induced interstitial lung disease during cancer therapies: expert opinion on diagnosis and treatment. ESMO Open 2022;7:100404. [Crossref] [PubMed]
- Kim S, Lim JU. Immune checkpoint inhibitor-related interstitial lung disease in patients with advanced non-small cell lung cancer: systematic review of characteristics, incidence, risk factors, and management. J Thorac Dis 2022;14:1684-95. [Crossref] [PubMed]
- Boku N, Ryu MH, Kato K, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol 2019;30:250-8. [Crossref] [PubMed]
- Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:234-47. [Crossref] [PubMed]
- Dai Y, Liu Y, Gong Z, et al. Revalidation of the ATTRACTION-4 study in a real-world setting: a multicenter, retrospective propensity score matching study in China. Front Immunol 2023;14:1264929. [Crossref] [PubMed]
- Shitara K, Munakata M, Koizumi W, et al. A case of suspected S-1 induced interstitial pneumonia. Gan To Kagaku Ryoho 2007;34:619-22. [PubMed]
- Ishizone S, Koide N, Akita N, et al. Fatal interstitial pneumonia associated with oxaliplatin-based therapy in a patient with metastatic rectal cancer. Clin J Gastroenterol 2011;4:157-61. [Crossref] [PubMed]
- Nakahama K, Tamiya A, Taniguchi Y, et al. Severe acute interstitial lung disease after nivolumab in three non-small cell lung cancer patients with imaging findings of airway obstruction adjacent to lung tumors. J Infect Chemother 2017;23:826-9. [Crossref] [PubMed]
- Uhara H, Tsuchida T, Kiyohara Y, et al. Safety and effectiveness of nivolumab in Japanese patients with malignant melanoma: Final analysis of a post-marketing surveillance. J Dermatol 2022;49:862-71. [Crossref] [PubMed]
- Khoja L, Day D, Wei-Wu Chen T, et al. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol 2017;28:2377-85. [Crossref] [PubMed]
- Ando T, Ueda A, Ogawa K, et al. Prognosis of Immune-related Adverse Events in Patients With Advanced Gastric Cancer Treated With Nivolumab or Pembrolizumab: A Multicenter Retrospective Analysis. In Vivo 2021;35:475-82. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Management of Immunotherapy-Related Toxicities. Version 1. 2022. February 28, 2022.
- Camard M, Besse B, Cariou PL, et al. Prevalence and outcome of steroid-resistant/refractory pneumonitis induced by immune checkpoint inhibitors. Respir Med Res 2022;82:100969. [Crossref] [PubMed]
- Lai KA, Sheshadri A, Adrianza AM, et al. Role of Infliximab in Immune Checkpoint Inhibitor-Induced Pneumonitis. J Immunother Precis Oncol 2020;3:172-4. [Crossref] [PubMed]
- Shimoji K, Masuda T, Yamaguchi K, et al. Association of Preexisting Interstitial Lung Abnormalities With Immune Checkpoint Inhibitor-Induced Interstitial Lung Disease Among Patients With Nonlung Cancers. JAMA Netw Open 2020;3:e2022906. [Crossref] [PubMed]
- Nakanishi Y, Masuda T, Yamaguchi K, et al. Pre-existing interstitial lung abnormalities are risk factors for immune checkpoint inhibitor-induced interstitial lung disease in non-small cell lung cancer. Respir Investig 2019;57:451-9. [Crossref] [PubMed]
- Graur A, Montesi SB, Lanuti M, et al. Treating lung cancer in patients with interstitial lung disease: what do we know? J Thorac Dis 2023;15:1555-8. [Crossref] [PubMed]
- Kim S, Oh IJ, Park SY, et al. Corticosteroid therapy against treatment-related pulmonary toxicities in patients with lung cancer. J Thorac Dis 2014;6:1209-17. [PubMed]
- Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17. [Crossref] [PubMed]
- Ichikado K, Johkoh T, Ikezoe J, et al. Acute interstitial pneumonia: high-resolution CT findings correlated with pathology. AJR Am J Roentgenol 1997;168:333-8. [Crossref] [PubMed]
- Murohashi K, Hara Y, Aoki A, et al. Diffuse alveolar hemorrhage complicating acute exacerbation of IPF. Respir Med Case Rep 2020;29:101022. [Crossref] [PubMed]
- Gando S, Kameue T, Matsuda N, et al. Systemic inflammation and disseminated intravascular coagulation in early stage of ALI and ARDS: role of neutrophil and endothelial activation. Inflammation 2004;28:237-44. [Crossref] [PubMed]
- Sato R, Imamura K, Sakata S, et al. Disorder of Coagulation-Fibrinolysis System: An Emerging Toxicity of Anti-PD-1/PD-L1 Monoclonal Antibodies. J Clin Med 2019;8:762. [Crossref] [PubMed]
- Toi Y, Sugawara S, Kawashima Y, et al. Association of Immune-Related Adverse Events with Clinical Benefit in Patients with Advanced Non-Small-Cell Lung Cancer Treated with Nivolumab. Oncologist 2018;23:1358-65. [Crossref] [PubMed]
- Sato K, Akamatsu H, Murakami E, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 2018;115:71-4. [Crossref] [PubMed]
- Fuchs CS, Muro K, Tomasek J, et al. Prognostic Factor Analysis of Overall Survival in Gastric Cancer from Two Phase III Studies of Second-line Ramucirumab (REGARD and RAINBOW) Using Pooled Patient Data. J Gastric Cancer 2017;17:132-44. [Crossref] [PubMed]
- Matsuno O, Okubo T, Hiroshige S, et al. Drug-induced lymphocyte stimulation test is not useful for the diagnosis of drug-induced pneumonia. Tohoku J Exp Med 2007;212:49-53. [Crossref] [PubMed]
- Otsuka H, Sano A, Azuma Y, et al. Surgical lung biopsy for interstitial lung diseases-a single center study of 129 patients. J Thorac Dis 2022;14:1972-9. [Crossref] [PubMed]


