
BMP6 inhibits gastric cancer growth and predicts good prognosis
Highlight box
Key findings
• Bone morphogenetic protein 6 (BMP6) expression is correlated with the prognosis of gastric cancer (GC) patients and can simultaneously inhibit the proliferation of GC cells.
What is known and what is new?
• BMP6 is closely associated with the occurrence and development of various tumors.
• BMP6 can inhibit the growth and proliferation of GC cells through the nuclear factor-κB pathway.
What is the implication, and what should change now?
• BMP6 may be a new target for the treatment of GC.
Introduction
Gastric cancer (GC) is a common malignant tumor of digestive tract, and it is also the leading cause of death related to malignant tumor (1). Due to its often nonspecific early symptoms, GC is frequently diagnosed with lymph node or distant organ metastasis (2). In recent years, although chemotherapy has made some progress, the long-term survival rate of patients has not been significantly improved (3), and targeted therapies such as epidermal growth factor receptor (EGFR) inhibitors and mesenchymal-epithelial transition (MET) inhibitors, have also proven ineffective in treating GC (4).
Bone morphogenetic protein 6 (BMP6) is a member of the transforming growth factor-β ligand superfamily, which is mainly involved in tissue osteogenesis, differentiation and homeostasis, fetal development, neurogenesis, and iron metabolism (5). BMP6 plays a vital role in iron homeostasis and has been identified as the primary physiological regulator of hepcidin, affecting iron overload (6). It can also inhibit cell invasiveness by increasing cell-to-cell adhesion, and it has been shown to directly induce vascular endothelial cells and promote angiogenesis (7).
Abnormal BMP6 expression has been observed in various tumors, affecting multiple biological processes like cell growth, proliferation, invasion, metastasis, and immunity (8-11). However, the function and role of BMP6 in GC remain unclear. This paper reports on the biological role of BMP6 expression in GC tissue. We present this article in accordance with the MDAR and ARRIVE reporting checklists (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-512/rc).
Methods
Sample material
Tissue blocks from patients who underwent radical surgery for GC in the Department of General Surgery Qingpu Branch, Zhongshan Hospital, Fudan University, between April 2012 and January 2013 were selected, and clinical and pathological information of the patients was collected. A total of 79 patients were included, including 52 males and 27 females, with a minimum age of 37 years and a maximum age of 88 years, and a median age of 60 years. Inclusion criteria: (I) all were pathologically diagnosed with gastric adenocarcinoma; (II) no tumor-related treatment was received before surgery. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee of Qingpu Branch, Zhongshan Hospital, Fudan University (No. IEC-C-007-A08-V.03), and the enrolled patients signed an informed consent form before participation.
Tissue microarray production
The clinical pathological slides of the 79 enrolled patients were observed under a microscope, and the cancer cells and adjacent tissues were marked separately, and then the corresponding positions of the wax blocks were marked. The adjacent tissues and cancer tissues of the same patient were used as controls. Tissue microarrays were produced using the Shanghai Bonan tissue microarray spotter, and the tissue microarrays were scanned and scored using a high-throughput biological tissue analysis system. As of December 2019, the median overall survival of the entire group of patients was 43 months [95% confidence interval (CI): 13.5–72] in the follow-up.
Data acquisition
Download and organize RNA sequencing (RNA-seq) data processed using the STAR pipeline from The Cancer Genomics Atlas (TCGA) database (https://portal.gdc.cancer.gov) for the TCGA-stomach adenocarcinoma (STAD) project and extract the data in fragments per kilobase million (FPKM) format. Depending on the data format characteristics, choose between the Mann-Whitney U test (Wilcoxon rank sum test) or the paired sample t-test for statistical analysis. Visualize the data using the ggplot2 package.
Cell culture
GES normal gastric cell line and two GC cell lines, MGC803, A95, and SGC7901, were purchased from the cell bank of the Shanghai Branch of the Chinese Academy of Sciences. Dulbecco’s modified Eagle’s medium (DMEM) medium containing 10% fetal bovine serum and 1% double antibody (streptomycin-penicillin mixed solution) was used for cell culture, which was placed in a culture box at 37 ℃ and 5% CO2. The cells were observed under a microscope and were adherent cells.
We designed and constructed a BMP6 overexpression plasmid as well as two pairs of BMP6 RNA interference sequences [BMP6-short hairpin (sh)1 and BMP6-sh2]:
- BMP6-sh1-forward (F): CCGGCCACAAAGAGTTCAAGTTCAACTCGAGTTGAACTTGAACTCTTTGTGGTTTTTG;
- BMP6-sh1-reverse (R): AATTCAAAAACCACAAAGAGTTCAAGTTCAACTCGAGTTGAACTTGAACTCTTTGTGG;
- BMP6-sh2-F: CCGGCGCATCTACAAGGACTGTGTTCTCGAGAACACAGTCCTTGTAGATGCGTTTTTG;
- BMP6-sh2-R: AATTCAAAAACGCATCTACAAGGACTGTGTTCTCGAGAACACAGTCCTTGTAGATGCG.
The above shRNA sequences were synthesized and ligated into the PLKO.1 vector. GC cells were seeded in a six-well plate, and when they reached approximately 50% confluency, the culture medium was removed. The cells were then infected with viruses carrying BMP6 shRNA sequences using puromycin resistance for positive cell clone selection. This process resulted in stable cell lines with either BMP6 overexpression or knockdown. In the case of the overexpression plasmid, we designed it with a Flag tag for subsequent detection to confirm successful overexpression.
Western blotting analysis
Cells were washed 2–3 times with phosphate-buffered saline (PBS) and then lysed in an appropriate volume of radioimmunoprecipitation assay (RIPA) buffer (with added protease inhibitors) using a cell scraper. The cell lysate was transferred to a 1.5 mL centrifuge tube and placed on ice for 30 minutes before being centrifuged at 12,000 rpm, 4 ℃ for 10 minutes. The resulting supernatant was collected as the total protein solution. Protein concentration was determined using a bicinchoninic acid (BCA) protein assay kit following the manufacturer’s instructions. The protein solution was then mixed with reducing protein loading buffer, denatured in a boiling water bath for 15 minutes, and stored at −20 ℃ until further use. Equal amounts of protein were separated by 6–10% µ-polyacrylamide gel electrophoresis (PAGE), transferred onto a polyvinylidene fluoride (PVDF) membrane, and incubated with primary antibodies overnight at 4 ℃ after blocking with 5% non-fat milk in tris-buffered saline Tween-20 (TBST) for 1 hour at room temperature. The membrane was then cut into strips and incubated with secondary antibodies.
3-(4,5-dimethylthiazol-2-yl-)2,5-diphenyltetrazolium bromide (MTT) assay
Cell growth was measured by the MTT method in vitro. Cells were seeded in a 96-well plate at a density of 2,000 cells/well and cultured with 10% fetal bovine serum (FBS) in DMEM. MTT (5 mg/mL) was added to each well using a pipette and incubated in a cell culture incubator for 3 hours. The MTT was then removed, and the cells were dissolved in dimethyl sulfoxide (DMSO) for 10 days. The absorbance was measured with an enzyme-linked immunosorbent assay reader, and the results were expressed as the mean ± standard error of three independent experiments.
Crystal violet assay
The crystal violet assay was used to determine the clone formation ability of cells in vitro. Cells were seeded in a six-well plate at a density of 1,000 cells/well and cultured with 10% FBS in DMEM for 10 days. The cells were then stained with 0.5% crystal violet solution for 5 minutes, washed with PBS, and dried. The cells were photographed, and the absorbance was measured with a spectrophotometer to observe the cell growth.
Xenograft assay
The animal experimental protocol was approved by the Ethics Committee of Qingpu Branch, Zhongshan Hospital, Fudan University (No. IEC-C-007-A08-V.03), and followed the institutional Guidelines for the Care and Use of Laboratory Animals. A protocol was prepared before the study without registration. The cells were digested with trypsin and suspended in complete medium (containing 10% FBS) to make a cell suspension (cell concentration of about 2×106 cells/100 µL). Each nude mouse was injected subcutaneously with 100 µL of the cell suspension in the abdominal wall. After the experiment, the mice were euthanized by cervical dislocation, and the tumors were removed. The tumors were carefully separated using blunt dissection, and the tumor membrane was kept intact. The tumors were arranged in a certain order, photographed with a white background, and a ruler was placed next to them for reference.
Statistical analysis
All cellular experiments were conducted three times, and statistical comparisons were performed using the independent sample t-test. Experimental data are presented as the mean ± standard deviation, and differences between groups were compared using a t-test and analyzed using GraphPad Prism 9.0 software.
Results
BMP6 is low-expressed in GC tissues and is associated with poor prognosis in patients
We initially extracted gastric adenocarcinoma data from the TCGA database and conducted an analysis. The results revealed that, in comparison to normal tissues, BMP6 expression was significantly reduced in gastric adenocarcinoma tissues. We obtained the same results when comparing paired samples (Figure 1A,1B). To study the expression of BMP6 in tissues and its relationship with the clinical prognosis of GC patients, we created a relevant tissue microarray. The results showed that BMP6 expression was significantly lower in gastric adenocarcinoma tissues than in adjacent non-cancerous tissues (Figure 1C,1D). Further analysis of the relationship between BMP6 expression and clinical pathological characteristics of patients revealed that patients with low BMP6 expression (score ≥70) had a later pathological stage and were more prone to lymph node metastasis (χ2=8.385, P=0.004). In terms of pathological grading, they also had a higher malignant degree, while there was no significant correlation with gender, age, tumor size, presence of cancer embolus or nerve invasion (Table 1). At the same time, we analyzed the prognosis of the patients and found that low expression of BMP6 was associated with poor prognosis in patients (P value <0.05) (Figure 1E).

Table 1
| Characteristics | N | High expression group, n | Low expression group, n | χ2 | P |
|---|---|---|---|---|---|
| Age | 0.067 | 0.795 | |||
| >60 years | 32 | 10 | 22 | ||
| ≤60 years | 47 | 16 | 31 | ||
| Sex | 0.907 | 0.341 | |||
| Female | 27 | 2 | 20 | ||
| Male | 52 | 19 | 33 | ||
| Pathological grading | 12.897 | <0.01 | |||
| I, II | 27 | 16 | 11 | ||
| III | 52 | 10 | 42 | ||
| Cancer embolus | 1.398 | 0.237 | |||
| No | 64 | 23 | 41 | ||
| Yes | 15 | 3 | 12 | ||
| Nerve invasion | 0.066 | 0.798 | |||
| No | 72 | 24 | 48 | ||
| Yes | 7 | 2 | 5 | ||
| Tumor size | 1.229 | 0.268 | |||
| >50 cm2 | 28 | 7 | 21 | ||
| ≤50 cm2 | 51 | 19 | 32 | ||
| T stage | 3.078 | 0.079 | |||
| T1, T2 | 26 | 12 | 14 | ||
| T3, T4 | 53 | 14 | 39 | ||
| Lymphatic metastasis | 8.385 | 0.004 | |||
| No | 28 | 15 | 13 | ||
| Yes | 51 | 11 | 40 | ||
| Pathological stage | 7.905 | 0.005 | |||
| I, II | 43 | 20 | 23 | ||
| III, IV | 36 | 6 | 30 |
BMP6, bone morphogenetic protein 6.
BMP6 can inhibit the growth and proliferation of GC cells
We cultured GES normal GC cell line and MGC803, A95, SGC7901 GC cell lines to determine the expression of BMP6 protein in normal gastric mucosal epithelial cell lines and GC cell lines. We collected these four types of cells for protein lysis and used Western Blot to study the expression of BMP6 protein in GC cell lines, MGC803, and SGC7901. We found that BMP6 protein was expressed in all three cell lines, with relatively high expression in normal gastric mucosal cells and SGC7901 cell lines, and similar expression in other GC cell lines (Figure 2A). We separately constructed cell lines of MGC803 and SGC7901 with overexpressed and knocked down BMP6 expression (Figure 2B,2C).

We used MTT assay to study the effect of interference with endogenous BMP6 gene expression on the proliferation of GC cell lines. The results showed (Figure 2D) that the growth and reproduction of interference endogenous BMP6 expression group were faster than that of the blank control group in MGC803 and SGC7901 GC cell lines. We then performed crystal violet experiments to further validate the results, which were consistent with the MTT assay results (Figure 2E,2F). Similarly, in MGC803 and SGC7901 GC cell lines, the overexpression BMP6 group grew slower than the blank control group. We then performed crystal violet experiments to verify the results, which were consistent with the MTT assay results (Figure 2G-2I). We then conducted in vivo experiments to further speculate our hypothesis. We chose MGC803 cell line to further conduct in vivo tumor formation experiments, and found that interference BMP6 expression significantly promoted the growth of tumors in vivo compared with the control group (Figure 2J,2K). On the contrary, the growth of tumors in the overexpression BMP6 group was significantly smaller than that in the control group (Figure 2L,2M). These results indicate that BMP6 may play an important role in the development of GC.
BMP6 can activate the nuclear factor-κB (NF-κB) pathway to inhibit the proliferation and growth of GC cells
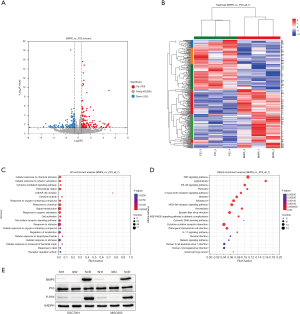
To further explore the potential mechanism of BMP6 inhibiting GC cell proliferation, we sequenced cells overexpressing BMP6 and normal controls. The results showed that compared with the control group, there were 130 upregulated genes and 152 downregulated genes after BMP6 overexpression (Figure 3A,3B). We further performed functional enrichment analysis on these 282 genes and found that the NF-κB pathway was significantly enriched (Figure 3C,3D). This caught our attention, and we further validated it by Western blot. We found that compared with normal cells, the expression of the key molecule p65 of the NF-κB pathway did not change significantly after knocking down BMP6 expression, but the expression of p-p65 was significantly reduced. This suggests that when BMP6 expression is inhibited, the activity of the NF-κB pathway is also significantly inhibited (Figure 3E). This indicates that BMP6 may exert its biological functions through the NF-κB pathway.
Discussion
GC is the fourth most common cancer worldwide and the third leading cause of cancer-related deaths (12). It is expected that these numbers will increase by 2040, with an estimated 10,000 new cases and 770,000 deaths globally (13). Although the incidence of GC has stabilized and declined in Europe and the United States in recent years, it continues to rise significantly in East Asia (14). Because early symptoms are not specific, GC is often diagnosed at an advanced stage, and once it metastasizes, the prognosis is poor (15). In recent years, research on molecular mechanisms and key signaling pathways has brought new opportunities and challenges for the diagnosis and treatment of GC.
In our study, survival analysis of 79 GC patients showed that BMP6 expression levels affected patient survival, and low BMP6 expression to some extent indicated poor prognosis, which is consistent with previous studies (16,17). Studies have also shown that in breast cancer, BMP6 acts as a tumor suppressor gene and plays an important role in proliferation, differentiation, and chemoresistance of breast cancer cells (18,19). Some scholars have used TCGA analysis to find that high BMP6 expression is associated with poor prognosis. However, in estrogen receptor-positive (ER+) malignant breast tumors, high BMP6 expression can enhance immune cell infiltration and has a better prognosis (8). We found that patients with low BMP6 expression levels had later pathological stages, poor prognosis, and were more likely to have lymph node metastasis (χ2=8.385, P=0.004), while BMP6 expression levels had no significant correlation with gender, age, tumor size, presence of cancer thrombus, or nerve invasion. Therefore, low BMP6 expression may promote the proliferation of gastric malignant tumors. This experiment also found that BMP6 gene expression levels were correlated with the T staging of primary tumors (χ2=3.078, P=0.079), which may be related to sample selection bias and small sample size.
Based on further experimental research, we found that interfering with BMP6 gene expression in MGC803 and SGC7901 cell lines significantly accelerated cell proliferation compared with the control group. Similarly, we selected stable overexpressing BMP6 protein cell lines in MGC803 and SGC7901 cell lines and found that overexpression of BMP6 gene significantly slowed down cell proliferation compared with the control group. Subsequently, we studied the relevant biology of BMP6 in animals, and further studied the role of BMP6 in GC cells. The results showed that knocking down BMP6 expression can significantly promote the growth of subcutaneous tumors in animals, while overexpression of BMP6 can significantly inhibit the growth of subcutaneous tumors. This suggests that BMP6 plays an important role in the occurrence and development of GC.
Through RNA-seq, we found that the NF-κB pathway was significantly enriched. Further validation showed that the expression of the key molecule p-p65 in the NF-κB pathway was significantly decreased when BMP6 expression was knocked down compared to normal cells, indicating that the activity of the NF-κB pathway was significantly suppressed when BMP6 expression was inhibited. The function and regulation of NF-κB have been widely studied. NF-κB plays a key and evolutionarily conserved role in regulating the immune system, causing rapid responses to pathogens, cell differentiation, and survival (20). Activation of the NF-κB signaling pathway leads to the upregulation of multiple genes responsible for subsequent inflammation and immune responses. Increasing evidence suggests that the dysregulation of the NF-κB signaling pathway may lead to the development of serious chronic inflammation, autoimmune diseases, and cancer (21-23), and there is also research showing its close relationship with GC (24,25).
Of course, this study also has some limitations. We did not use inhibitors to verify the NF-κB pathway in our analysis. In future experiments, we will inhibit the NF-κB pathway using inhibitors to further investigate the relationship between BMP6 and the development of GC.
Conclusions
In conclusion, our study found that BMP6 was significantly downregulated in GC tissue and affected patient prognosis. BMP6 gene expression may suppress the growth and proliferation of GC cells through the NF-κB signaling pathway, providing a new research direction for the prevention and treatment of GC.
Acknowledgments
Funding: This work was supported by grants from
Footnote
Reporting Checklist: The authors have completed the MDAR and ARRIVE reporting checklists. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-512/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-512/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-512/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-512/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee of Qingpu Branch, Zhongshan Hospital, Fudan University (No. IEC-C-007-A08-V.03), and the enrolled patients signed an informed consent form before participation. The animal experimental protocol was approved by the Ethics Committee of Qingpu Branch, Zhongshan Hospital, Fudan University (No. IEC-C-007-A08-V.03), and followed the institutional Guidelines for the Care and Use of Laboratory Animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mantziari S, St Amour P, Abboretti F, et al. A Comprehensive Review of Prognostic Factors in Patients with Gastric Adenocarcinoma. Cancers (Basel) 2023;15:1628. [Crossref] [PubMed]
- Li W, Ng JM, Wong CC, et al. Molecular alterations of cancer cell and tumour microenvironment in metastatic gastric cancer. Oncogene 2018;37:4903-20. [Crossref] [PubMed]
- Li Q, Xu X, Su D, et al. Long-term survival of an elderly patient with advanced gastric cancer after combination therapy: a case report and literature review. BMC Cancer 2019;19:459. [Crossref] [PubMed]
- Tirino G, Pompella L, Petrillo A, et al. What's New in Gastric Cancer: The Therapeutic Implications of Molecular Classifications and Future Perspectives. Int J Mol Sci 2018;19:2659. [Crossref] [PubMed]
- Silvestri L, Nai A, Dulja A, et al. Hepcidin and the BMP-SMAD pathway: An unexpected liaison. Vitam Horm 2019;110:71-99. [Crossref] [PubMed]
- Pizzamiglio S, De Bortoli M, Taverna E, et al. Expression of Iron-Related Proteins Differentiate Non-Cancerous and Cancerous Breast Tumors. Int J Mol Sci 2017;18:410. [Crossref] [PubMed]
- Zabkiewicz C, Resaul J, Hargest R, et al. Bone morphogenetic proteins, breast cancer, and bone metastases: striking the right balance. Endocr Relat Cancer 2017;24:R349-66. [Crossref] [PubMed]
- Mo YQ, Nakamura H, Tanaka T, et al. Lysosomal exocytosis of HSP70 stimulates monocytic BMP6 expression in Sjögren's syndrome. J Clin Invest 2022;132:e152780. [Crossref] [PubMed]
- Min D, Byun J, Lee EJ, et al. Epigenetic Silencing of BMP6 by the SIN3A-HDAC1/2 Repressor Complex Drives Melanoma Metastasis via FAM83G/PAWS1. Mol Cancer Res 2022;20:217-30. [Crossref] [PubMed]
- Katsuta E, Maawy AA, Yan L, et al. High expression of bone morphogenetic protein (BMP) 6 and BMP7 are associated with higher immune cell infiltration and better survival in estrogen receptor-positive breast cancer. Oncol Rep 2019;42:1413-21. [Crossref] [PubMed]
- Xiong W, Wang L, Yu F. Expression of bone morphogenetic protein 6 in non-small cell lung cancer and its significance. Oncol Lett 2019;17:1946-52. [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: An overview. Int J Cancer 2021; Epub ahead of print. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ajani JA, Lee J, Sano T, et al. Gastric adenocarcinoma. Nat Rev Dis Primers 2017;3:17036. [Crossref] [PubMed]
- He Y, Cui Y, Xu B, et al. Hypermethylation leads to bone morphogenetic protein 6 downregulation in hepatocellular carcinoma. PLoS One 2014;9:e87994. [Crossref] [PubMed]
- Wang Q, Vattai A, Vilsmaier T, et al. Immunogenomic Identification for Predicting the Prognosis of Cervical Cancer Patients. Int J Mol Sci 2021;22:2442. [Crossref] [PubMed]
- Liu G, Liu YJ, Lian WJ, et al. Reduced BMP6 expression by DNA methylation contributes to EMT and drug resistance in breast cancer cells. Oncol Rep 2014;32:581-8. [Crossref] [PubMed]
- Luo X, Wang G, Wang Y, et al. Gibberellin derivative GA-13315 overcomes multidrug resistance in breast cancer by up-regulating BMP6 expression. Front Pharmacol 2022;13:1059365. [Crossref] [PubMed]
- Guldenpfennig C, Teixeiro E, Daniels M. NF-kB's contribution to B cell fate decisions. Front Immunol 2023;14:1214095. [Crossref] [PubMed]
- Aqdas M, Sung MH. NF-κB dynamics in the language of immune cells. Trends Immunol 2023;44:32-43. [Crossref] [PubMed]
- Zhang L, Sun L, Wang L, et al. Mitochondrial division inhibitor (mdivi-1) inhibits proliferation and epithelial-mesenchymal transition via the NF-κB pathway in thyroid cancer cells. Toxicol In Vitro 2023;88:105552. [Crossref] [PubMed]
- Gado F, Ferrario G, Della Vedova L, et al. Targeting Nrf2 and NF-κB Signaling Pathways in Cancer Prevention: The Role of Apple Phytochemicals. Molecules 2023;28:1356. [Crossref] [PubMed]
- Zhu S, Al-Mathkour M, Cao L, et al. CDK1 bridges NF-κB and β-catenin signaling in response to H. pylori infection in gastric tumorigenesis. Cell Rep 2023;42:112005. [Crossref] [PubMed]
- Wang MQ, Chen YR, Xu HW, et al. HKDC1 upregulation promotes glycolysis and disease progression, and confers chemoresistance onto gastric cancer. Cancer Sci 2023;114:1365-77. [Crossref] [PubMed]


