
The long-term outcomes and prognostic factors about locally advanced right colon cancer: a retrospective cohort study
Highlight box
Key findings
• En bloc resection of locally advanced right-sided colon cancers can achieve significant survival benefits for these patients.
What is known and what is new?
• Right-sided colon cancers invading the pancreas and duodenum are rare, technically complicated, and have unknown oncologic consequences.
• En bloc resection is a viable management option in patients with locally advanced right-sided colon cancer.
What is the implication, and what should change now?
• En bloc resection may help improve prognosis, especially in patients without local nodal involvement. Large sample multicenter studies may yield more reliable results.
Introduction
Colorectal cancer ranks third in incidence and second in mortality among malignancies worldwide (1). Locally advanced colorectal cancers invading into adjacent organs account for 5.5–16.7% of all colorectal cancers (2,3). Although a tumour located in the right side of colon has less invasive potential than one in the left colon, 0.9–2.6% of right-sided colon cancers are found to involve adjacent organs at diagnosis (4-6). In particular, right-sided colon cancer with direct infiltration of the duodenum and/or pancreatic head is usually associated with higher morbidity and often pose a surgical challenge.
Currently, a curative resection is considered the first choice for definitive treatment for locally advanced colorectal cancer, with 5-year survival rates between 43% to 83% after radical resection (7-10). Perineural invasion, poorly differentiated tumor, regional lymph node dissemination, microsatellite instability (MSI) status, pancreatic invasion and no perioperative chemotherapy were considered the significant predictors of poor survival. Most of the previous studies suffer from small sample sizes and simple statistical methods (7-10). However, achieving R0 resection is paramount in these high-risk patients, as a positive tumor margin (R1) is a poor prognostic factor and increases the risk of recurrence, requiring multi-visceral en bloc resection (11). The first case of treatment with en bloc right hemicolectomy with pancreatoduodenectomy (RHCPD) for locally advanced right-sided colon cancer (LARCC) invading the pancreas, duodenum, or other organs, was reported in 1953 by Van Prohaska (12). RHCPD is an effective way to achieve a R0 margin for LARCC. Right-sided colon cancers invading the pancreas and duodenum are rare, technically complex, and with unknown oncologic consequences, so there are few reports on the survival status and factors associated with survival in this category of right-sided colon cancer. The aim of this study was to report our experience of en bloc resection for LARCC and the long-term survival and risk factors associated with survival of these cases. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-928/rc).
Methods
Patient characteristics
The database at Affiliated Cancer Hospital of Zhengzhou University was reviewed from January 2003 to January 2022. During this period, 6,320 patients treated with right hemicolectomy for cancer were admitted to our department (Department of General Surgery, Affiliated Cancer Hospital of Zhengzhou University). Among them, 58 cases underwent en bloc resection due to preoperative imaging and intraoperative exploration showing tumour invasion of the duodenum and/or other organs. Postoperative pathologic findings in 47 patients determined that the adhesions between organs were malignant. We excluded patients whose postoperative pathology suggested only inflammatory adhesions in the expectation that we could explore the survival of patients with locally advanced tumors in the presence of tumors invading other organs who had undergone en bloc resection. The criteria for inclusion of patients were as follows: (I) preoperative or postoperative histological confirmation that the primary tumour site was the right colon; (II) preoperative imaging evaluation confirmed that the tumour was amenable to radical resection (tumor does not invade important blood vessels, such as the superior mesenteric artery and portal vein); (III) there was no distant metastasis of the tumour; and (IV) histological findings of the tumour confirmed that the tumour was not an inflammatory adhesion, but a direct invasion of the surrounding organs. We directly removed the samples with missing data. We excluded patients who had distant metastases from the tumour and those whose tumour primary lesion was not in the right colon. Computed tomography (CT) was routinely performed before surgery in all patients to determine local tumour infiltration of the tumour. Colonoscopy and histopathological determination of the tumour was conducted prior to surgery to confirm the histological origin of the tumour. When CT fails to identify malignant adhesions, magnetic resonance imaging (MRI) and endoscopic ultrasound (EUS) should be considered for further differentiation. If whole-body CT reveals suspicious distant metastatic lesions, positron emission tomography/CT (PET-CT) should be performed for differentiation. In our study, neoadjuvant chemotherapy (NAC) was performed in all the patients included in the later part of the study when their physical condition could tolerate it. Because of the time span of the study and the many controversies about NAC for colon cancer in the pre-study period, surgery was performed directly without NAC.
Because this study is an observational cohort study and a study of a relatively rare procedure, we included as many appropriate samples as possible so that we could better dilute some possible patient selection bias. The primary outcome was the overall survival after surgery. The secondary endpoints of the study included 30-day postoperative mortality, postoperative complications, prognostic factors, and tumour genetics. We classified the complications according to the Clavien-Dindo classification, with grade III and IV complications being defined as major morbidity. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University (No. 2018095). Due to the retrospective nature of the study and because no patient specimens were used, the requirement for individual consent for this retrospective analysis was waived by the ethics committee.
RHCPD technique
All patients underwent open surgery. The incision is made 1 cm to the right of the midline of the abdomen, with 2/3 of the incision located above the umbilicus. Depending on the size of the patient, the length of the incision ranged from approximately 15 to 25 centimeters. The vascular arch of the gastric omentum and the vessels of the lesser curvature of the stomach are first freed and separated, followed by mobilisation of the gastric antrum. The peri-mesenteric vessels of the transverse colon are transected, and the transverse colon is dissected. A standard cholecystectomy is performed, and the distal end of the common bile duct is dissected. Following ligation of the gastroduodenal artery, the ileum is divided approximately 20 cm from the ileocecal valve, and the ileocolic artery and the right colic artery are ligated in sequence. The gastrocolic venous trunk and the right branch of the middle colic artery are dissected and ligated to expose the portal vein and the distal end of the superior mesenteric artery. The posterior hiatus of the pancreas is released, and the neck of the pancreas is transected. The head of the pancreas is then flipped, and adhesiolysis is performed to separate the tumour from the pancreas. A space between the uncinate process of pancreas and the superior mesenteric vein (SMV) is created. Finally, the duodenum is disconnected at the horizontal portion. Reconstruction is then performed in the following order: pancreatic-enteric anastomosis, biliary-enteric anastomosis, and jejuno-jejunal-enteric anastomosis with a stapled side-side ileocolic anastomosis.
Contraindications to surgery include: (I) presence of distant organ metastases; (II) secondary involvement of the pancreatic head and/or duodenum, but not direct infiltration; (III) presence of an implanted cancerous nodule in the abdominal cavity; (IV) invasion of the hepatic artery, celiac trunk, or superior mesenteric artery; and (V) Nutritional Risk Screening 2002 (NRS 2002) score ≥3.
Follow-up
All patients were followed up with postoperative imaging at an interval of 3 months for the first 3 years and at an interval of 6 months for the next 2 years, and annual follow-up thereafter. Each follow-up visit consisted of a CT scan of the entire body and quantitative testing of tumour markers, including carcinoembryonic antigen (CEA), cancer antigen 19-9 (CA19-9), and cancer antigen 72-4 (CA72-4). Colonoscopy is performed 1 year after surgery, and each colonoscopy is repeated within 1 year if a progressive adenoma (villous adenoma larger than 1 cm in diameter, or high-grade atypical hyperplasia) is detected; if no progressive adenoma is detected, it is repeated within 3 years, and then every 5 years.
Statistical analysis
Data were analyzed using SPSS 25.0 statistical software (IBM Corp., Armonk, NY, USA). Quantitative variables were transformed into dichotomous variables for analysis and expressed as counts and percentages. The transformation of quantitative variables into dichotomous variables reduces the complexity of data processing, makes it easier to explain how the variables affect survival, and allows for a better visual presentation and a clearer characterization of the data. Overall survival was calculated using the Kaplan-Meier method, and survival outcomes were compared using the log-rank test. A value of P<0.05 was considered significant. Factors found to be significant in univariate analysis (P<0.05) were subjected to multivariate analysis. We performed variable screening and applied Cox regression models to model the relationship between survival time and the independent variables. Finally, we estimated the regression coefficients using maximum likelihood estimation and calculated the corresponding risk ratios and confidence intervals.
Results
There were 47 patients (23 males, 24 females) who underwent en bloc resection for LARCC. R0 resection was achieved in all cases. The clinical details of these patients are shown in Table 1. The median age of the patients was 61 years (range, 38–80 years). Nine patients had preoperative anemia and four patients underwent emergency surgery, three of whom had preoperative colonic obstruction and one of whom had preoperative tumour perforation. No patients had signs of distant metastasis on preoperative imaging. 22 patients had their CEA and CA19-9 levels tested 1 week before surgery, and 21 patients had their preoperative CA72-4 levels tested. The median preoperative value for CEA was 5.31 IU/mL (range, 0.81–167.2 IU/mL), that of CA19-9 was 16.64 IU/mL (range, 0.6–417.7 IU/mL), and that of CA72-4 was 3.48 IU/mL (range, 0.67–239.7 IU/mL). A total of 26 patients were treated preoperatively, 21 patients received 4 cycles of XELOX (capecitabine + oxaliplatin) regimen, 3 patients received 2 cycles of FOLFOX6 (fluorouracil + leucovorin + oxaliplatin) regimen, and 1 patient was treated with bevacizumab and irinotecan in combination with XELOX. Patients who underwent neoadjuvant therapy had better survival than those who did not (P=0.029).
Table 1
| Parameter | Values |
|---|---|
| Age (years) | 61 [38–80] |
| Gender | |
| Male | 23 (48.9) |
| Female | 24 (51.1) |
| Maximal tumour diameter (cm) | 8.5 [2.5–20.0] |
| CEA (IU/mL) | 5.31 [0.81–167.2] |
| CA19-9 (IU/mL) | 16.64 [0.6–417.7] |
| CA72-4 (IU/mL) | 3.48 [0.67–239.7] |
| Operative time (min) | 240 [140–400] |
| Operative blood loss (mL) | 450 [100–2,000] |
| Invasion of at least two organs | |
| Yes | 33 (71.4) |
| No | 14 (24.3) |
| Tumour differentiation | |
| Poor | 23 (48.9) |
| Moderate/well | 24 (51.1) |
| Perineural invasion | |
| Yes | 20 (42.6) |
| No | 27 (57.4) |
| Vascular invasion | |
| Yes | 22 (46.8) |
| No | 25 (53.2) |
| Regional lymph node | |
| Positive | 17 (36.2) |
| Negative | 30 (63.8) |
| Preoperative treatment | |
| Presence | 26 (55.3) |
| Absence | 21 (44.7) |
| MSI status | |
| MSI-H | 9 (19.1) |
| MSS | 12 (25.5) |
| KRAS | |
| Wild | 9 (50.0) |
| Mutant | 9 (50.0) |
| Complications | |
| Pancreatic leakage | 3 (23.1) |
| Abdominal infection | 2 (15.4) |
| Chyle leak | 7 (53.8) |
| Delayed gastric emptying | 1 (7.7) |
| Overall complication | 13 (27.7) |
| Major complication | 5 (10.6) |
| Perioperative mortality | 2 (4.3) |
Data are shown as median [range] or n (%). CEA, carcinoembryonic antigen; CA19-9, cancer antigen 19-9; CA72-4, cancer antigen 72-4; MSI, microsatellite instability; MSI-H, microsatellite instability-high; MSS, microsatellite instability-stable; KRAS, Kirsten rat sarcoma viral oncogene homolog.
The overall survival was 80.9%, 63.5%, and 51.7% at 1, 3, and 5 years, respectively (Figure 1). The average follow-up time was 80 months. At the follow-up cutoff date, 19 patients were still alive. Univariate survival analysis identified pancreatic invasion, regional lymph node positivity, more than two organs invaded, and no preoperative treatment as predictors of poor survival (log-rank P<0.05) (Table 2 and Figure 2). Subsequent multivariate analysis of these factors showed that regional lymph node positivity [95% confidence interval (CI): 1.145–7.736; P=0.025] and more than two organs invaded (95% CI: 1.321–26.981; P=0.020) were predictors of poor survival (Table 3). Postoperative histology confirmed adenocarcinoma of colonic origin in all patients. Tumour invasion of the pancreas was found in 15 cases. Tumour invasion of at least two organs was observed in 33 patients. There were 23 cases of poorly differentiated adenocarcinoma, 14 cases of moderately differentiated adenocarcinoma, and 10 cases of highly differentiated adenocarcinoma. A total of 18 patients underwent Kirsten rat sarcoma viral oncogene homolog (KRAS) gene testing, of which 9 patients (50%) were mutant. Twenty-one patients underwent MSI gene testing, and 9 patients were identified with microsatellite instability-high (MSI-H) status.
Table 2
| Prognostic factor | P value |
|---|---|
| Age (<60/≥60 years) | 0.661 |
| Gender (male/female) | 0.645 |
| Perineural invasion (yes/no) | 0.492 |
| Vascular invasion (yes/no) | 0.358 |
| Tumour differentiation (poor/moderate and well) | 0.770 |
| Pancreatic invasion (yes/no) | 0.027 |
| Invasion of at least two organs (yes/no) | 0.004 |
| Regional lymph node (positive/negative) | 0.003 |
| CEA (<5/≥5 IU/mL) | 0.117 |
| CA19-9 (<37/≥37 IU/mL) | 0.816 |
| CA72-4 (<8/≥8 IU/mL) | 0.858 |
| Preoperative treatment (presence/absence) | 0.029 |
| MSI status (MSI-H/MSS) | 0.281 |
| KRAS (wild/mutant) | 0.867 |
CEA, carcinoembryonic antigen; CA19-9, cancer antigen 19-9; CA72-4, cancer antigen 72-4; MSI, microsatellite instability; MSI-H, microsatellite instability-high; MSS, microsatellite instability-stable; KRAS, Kirsten rat sarcoma viral oncogene homolog.
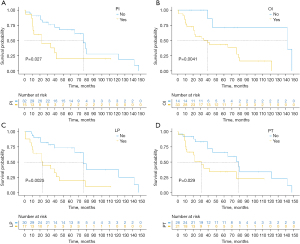
Table 3
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Pancreatic invasion | 1.327 | 0.457–3.853 | 0.603 |
| Invasion of at least two organs | 5.970 | 1.321–26.981 | 0.020 |
| Regional lymph node | 2.976 | 1.145–7.736 | 0.025 |
| Preoperative treatment | 1.422 | 0.584–3.464 | 0.438 |
HR, hazard ratio; CI, confidence interval.
The median diameter of the tumour was 8.5 (range, 2.5–20.0) cm, the median operative time was 240 (range, 140–400) minutes, the median operative blood loss was 450 (range, 100–2,000) mL, and the mean postoperative hospital stay was 26.0±7.2 days. All patients were cleared of local lymph nodes (mesenteric lymph nodes), the median number of lymph nodes cleared was 37 (range, 11–59), and a total of 17 patients showed positive local lymph nodes on postoperative pathology. Two patients died within 30 days of surgery. These two patients had preoperative bowel obstruction, which did not allow for adequate bowel preparation before surgery. Subsequently, an anastomotic leak developed postoperatively, causing intraabdominal sepsis and ultimately death from septic shock. Thirteen patients (27.7%) had postoperative complications, five of which were serious (grade >3), while three patients had pancreatic leaks, and two had abdominal infections. A total of seven patients experienced a mild chyle leak that was managed non-operatively, and one patient had delayed gastric emptying.
Discussion
Earlier research on LARCC have suggested that these patients have a poor prognosis and are considered unsuitable for resection because of the complexity of the surgery required. The 5-year survival following palliative resection for LARCC has been reported as low as 0–23% (7). However, with advances in surgical techniques, en bloc resection for LARCC is becoming accepted as a safe procedure in appropriate high volume tertiary centres (7,8,12). In previous reports in the literature, the 5-year survival rate after R0 resection in patients with LARCC ranged from 43% to 83.3% (7-10). The 5-year survival of our patient cohort is 51.7%. In our study, those patients with proven inflammatory adhesions on histological findings were excluded in order to more accurately characterize the prognosis of patients with LARCC after en bloc resection. A cautious approach should be taken as to whether the invasion is a malignant adhesion or not. Abdominal CT examination needs to focus on the clarity of the fat line between the right colon tumor and the duodenum, and the blurring or disappearance of the fat line needs to be taken into account for the possibility of malignant invasion. For patients who are difficult to identify, additional MRI and EUS should be considered. MRI can observe the relationship between colon tumor and surrounding tissues from multiple angles, and it is also more sensitive to fatty tissues. In addition, EUS is a good choice for patients who are difficult to identify. An ultrasound probe is placed on the tumor surface and scanned to determine the depth and extent of tumor infiltration and its relationship with surrounding adjacent organs, as well as to determine surrounding vascular and lymph node invasion. Duodenoscopy can also help in the identification, and can scrutinize the intestinal mucosa for signs of inflammation, depressions, sinus tracts, and other signs of invasion. Although advances in imaging technology have allowed us to stage the cancer and identify potential invasion of adjacent organs before surgery, it can still be challenging to clarify whether the tumour is infiltrating surrounding organs or simply adherent via an inflammatory adhesion without intraoperative exploration (13). Postoperative histological findings indicate that 33–94% (14-16) of the adhesions are in fact malignant. The percentage of preoperative assessment of malignant adhesions and postoperative pathologic findings suggestive of inflammatory adhesions was 18.9% in this study, and this percentage may decrease as preoperative screening techniques continue to improve. Intraoperatively, blind separation of these adhesions is associated with a high recurrence rate (90–100%), intraoperative tumour rupture, and a 5-year postoperative survival rate of only 17% (4). In the face of patients with suspected malignant invasion, it seems to us more sensible to perform en bloc resection as opposed to performing isolation.
In a study by Saiura et al. (14), pancreatic invasion was considered a poor prognostic factor. Another study series (7) demonstrated that metastatic abdominal wall spread and infiltration of the liver and gallbladder were independent factors affecting patient survival. In our study, the results of the univariate analysis showed that pancreatic invasion and invasion of more than two organs were independent predictors of poor prognosis, but in the results of the multifactorial analysis, only invasion of more than two organs was associated with a poorer prognosis.
One study (17) showed that neoadjuvant radiotherapy combined with total resection improved the R0 resection rate. Neoadjuvant chemotherapy can also reduce the stage of the primary tumour. Although the results of several studies (18,19) have shown that neoadjuvant chemotherapy or radiotherapy is indeed effective in promoting tumour regression in locally advanced colon cancer, few studies to date have demonstrated that preoperative chemotherapy can be used as a prognostic predictor in extended organ resection procedures. For stage T4b colon cancer, the current standard treatment strategy is surgery followed by chemotherapy (20). The National Comprehensive Cancer Network (NCCN) guidelines state that NAC may be considered for patients identified with stage T4B tumors without distant metastases. The European Society for Medical Oncology (ESMO) recommends the inclusion of neoadjuvant therapy in clinical studies. This study has shown that patients who did not receive neoadjuvant therapy had poorer survival (P=0.029), but we remain cautious about this result because this study is a small sample retrospective study. It has been controversial whether neoadjuvant therapy should be performed for locally advanced colon cancer.
It was noted that NAC did not significantly improve the grade of tumor regression in patients with cT3 and cT4b stage (18). The clinical study of the FOLFIXIRI, which enrolled a total of 23 patients with cT4N2M0 stage, found that NAC resulted in a significant reduction in tumor size (21). The most recent results published in the FOxTROT study seem to provide more confidence in neoadjuvant treatment of colon cancer (22). Patients in the NAC group had a lower recurrence rate and had better tumor regression and disease downgrading, with a significantly higher R0 resection rate than the control group.
In some studies (8,23), patients with MSI-H had a poorer prognosis. In our study, 42.9% of patients who underwent MSI gene testing were identified with MSI-H status, much greater than the overall frequency of MSI-H in patients with sporadic right-sided colon cancer (14.5%) (24). Our results correlate with a similar study involving LARCC patients in a high-volume tertiary center in the United Kingdom (10), which suggested that due to later presentation and thereby increased tumour progression, LARCC may be more likely to be MSI-H from defective DNA mismatch repair. In the FOxTROT study (22), patients with mismatch repair-deficient (dMMR) had a lower percentage of tumor regression than patients with mismatch repair-proficient (pMMR) (P<0.001). A clinical study (25) in 2015 found that patients with MSI-H could significantly benefit from the programmed cell death protein 1 (PD-1) antibody pembrolizumab. Whether preoperative immunotherapy in patients with LARCC with MSI-H results in more significant tumour regression outcomes and longer survival should be determined in follow-up studies.
Several studies and meta-analyses (7,8,26,27) have reported that local lymph node negativity is a predictor of good prognosis after en bloc resection in patients with LARCC. Previous studies (7,26,27) reported lymph node involvement in 42.9–51.9% of locally advanced adenocarcinomas; in our study, only 36.1% of patients were local lymph node-positive. This may be a discrepancy due to the small sample size. In our study, the 3-year overall survival was 78.4% in patients with local lymph node negativity, and only 38.8% in patients with positive local lymph nodes. In a multivariate survival analysis, we found that local lymph node negativity was a significant predictive factor for improved survival.
In terms of perioperative complications, whilst 27.7% of patients experienced postoperative complications, only five patients had serious complications (Clavien-Dindo Grade >3), two patients died within 30 days after surgery, the immediate cause of death for both of these patients was septic shock secondary to anastomotic leak. The presence of preoperative intestinal obstruction and colonic anastomotic contamination were important contributors to this outcome. Overall, the incidence of postoperative complications was acceptable in the context of a long-term oncological benefit from RHCPD. Therefore, RHCPD should be considered a feasible option for definitive management of patients with LARCC. Postoperative complications are also worth considering, and the novel “Huscher technique” has been proposed as a new strategy to ensure the safety of intraoperative pancreatic-enteric anastomosis and reduce the incidence of postoperative pancreatic fistulae (28).
For resectable locally advanced right hemicolon cancer, there are still many issues that deserve to be investigated, not only in terms of patient survival, but also advances in surgical techniques. Laparoscopic en bloc resection and transvaginal specimen extraction have been reported in relevant cases (29), and the advancement of surgical techniques may lead to fewer complications and shorter hospitalization. Laparoscopic complete mesocolic excision (CME) in right-sided colonic cancer resections has a higher number of lymph node dissection than open surgery and has shorter hospitalization days (30), exploring advances in surgical technique and performing laparoscopic resection of locally advanced right hemicolon cancer when R0 resection is possible is a direction for our subsequent research. This may reduce our concerns when choosing en bloc resection surgery. For patients without R0 resection, in addition to systemic therapy, pelvic perfusion therapy, which can be performed in the field of rectal cancer, can also provide us with new treatment ideas (31).
Some limitations of our study should be noted. Although the sample size of this study was relatively larger compared to previous studies, the sample size was still small and single center in nature. Due to the scarcity of such cases, it is difficult to conduct studies with large sample sizes. Moreover, most of such studies are retrospective, and there are some differences in results between countries and centers due to different patient selection criteria, among other factors. Some of the findings still need to be confirmed in large-sample, multicenter, prospective studies. The present study excluded patients with only inflammatory adhesions, and reflects the prognosis and treatment outcomes of patients with locally advanced right sided colon cancer with histologically proven multivisceral invasion treated with hemicolectomy and en bloc resection of affected organs. Our findings support the use of en bloc resection in cases of LARCC.
Conclusions
En bloc resection is highly effective in treating patients with LARCC. This aggressive approach may help improve prognosis, especially in patients without local nodal disease. Larger sample size, multicenter studies may yield more illuminating results.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-928/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-928/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-928/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-928/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University (No. 2018095). Due to the retrospective nature of the study and because no patient specimens were used, the requirement for individual consent for this retrospective analysis was waived by the ethics committee.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Staniunas RJ, Schoetz DJ Jr. Extended resection for carcinoma of colon and rectum. Surg Clin North Am 1993;73:117-29. [Crossref] [PubMed]
- Gebhardt C, Meyer W, Ruckriegel S, et al. Multivisceral resection of advanced colorectal carcinoma. Langenbecks Arch Surg 1999;384:194-9. [Crossref] [PubMed]
- Fuks D, Pessaux P, Tuech JJ, et al. Management of patients with carcinoma of the right colon invading the duodenum or pancreatic head. Int J Colorectal Dis 2008;23:477-81. [Crossref] [PubMed]
- Lee WS, Lee WY, Chun HK, et al. En bloc resection for right colon cancer directly invading duodenum or pancreatic head. Yonsei Med J 2009;50:803-6. [Crossref] [PubMed]
- Zhang J, Leng JH, Qian HG, et al. En bloc pancreaticoduodenectomy and right colectomy in the treatment of locally advanced colon cancer. Dis Colon Rectum 2013;56:874-80. [Crossref] [PubMed]
- Zhao YZ, Han GS, Lu CM, et al. Right hemicolectomy and multivisceral resection of right colon cancer: A report of 21 cases. J Huazhong Univ Sci Technolog Med Sci 2015;35:255-8. [Crossref] [PubMed]
- Yan XL, Wang K, Bao Q, et al. En bloc right hemicolectomy with pancreatoduodenectomy for right-sided colon cancer invading duodenum. BMC Surg 2021;21:302. [Crossref] [PubMed]
- Cojocari N, Crihana GV, Bacalbasa N, et al. Right-sided colon cancer with invasion of the duodenum or pancreas: A glimpse into our experience. Exp Ther Med 2021;22:1378. [Crossref] [PubMed]
- Das B, Fehervari M, Hamrang-Yousefi S, et al. Pancreaticoduodenectomy with right hemicolectomy for advanced malignancy: a single UK hepatopancreaticobiliary centre experience. Colorectal Dis 2023;25:16-23. [Crossref] [PubMed]
- Kumamoto T, Yamaguchi S, Nakagawa R, et al. Prognostic risk factors for pT4 colon cancer: A retrospective cohort study. Oncol Lett 2022;25:29. [Crossref] [PubMed]
- Van Prohaska J, Govostis MC, Wasick M. Multiple organ resection for advanced carcinoma of the colon and rectum. Surg Gynecol Obstet 1953;97:177-82. [PubMed]
- Kaneda Y, Noda H, Endo Y, et al. En bloc pancreaticoduodenectomy and right hemicolectomy for locally advanced right-sided colon cancer. World J Gastrointest Oncol 2017;9:372-8. [Crossref] [PubMed]
- Saiura A, Yamamoto J, Ueno M, et al. Long-term survival in patients with locally advanced colon cancer after en bloc pancreaticoduodenectomy and colectomy. Dis Colon Rectum 2008;51:1548-51. [Crossref] [PubMed]
- Kapoor S, Das B, Pal S, et al. En bloc resection of right-sided colonic adenocarcinoma with adjacent organ invasion. Int J Colorectal Dis 2006;21:265-8. [Crossref] [PubMed]
- Sheng QS, Chen WB, Li MJ, et al. Combined right hemicolectomy and pancreaticoduodenectomy for locally advanced right hemicolon cancer. Hepatobiliary Pancreat Dis Int 2015;14:320-4. [Crossref] [PubMed]
- Cukier M, Smith AJ, Milot L, et al. Neoadjuvant chemoradiotherapy and multivisceral resection for primary locally advanced adherent colon cancer: a single institution experience. Eur J Surg Oncol 2012;38:677-82. [Crossref] [PubMed]
- Dehal A, Graff-Baker AN, Vuong B, et al. Neoadjuvant Chemotherapy Improves Survival in Patients with Clinical T4b Colon Cancer. J Gastrointest Surg 2018;22:242-9. [Crossref] [PubMed]
- Liu F, Yang L, Wu Y, et al. CapOX as neoadjuvant chemotherapy for locally advanced operable colon cancer patients: a prospective single-arm phase II trial. Chin J Cancer Res 2016;28:589-97. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Colon Cancer. Version 4.2023. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1428
- Zhou H, Song Y, Jiang J, et al. A pilot phase II study of neoadjuvant triplet chemotherapy regimen in patients with locally advanced resectable colon cancer. Chin J Cancer Res 2016;28:598-605. [Crossref] [PubMed]
- Morton D, Seymour M, Magill L, et al. Preoperative Chemotherapy for Operable Colon Cancer: Mature Results of an International Randomized Controlled Trial. J Clin Oncol 2023;41:1541-52. [Crossref] [PubMed]
- Yamanashi T, Nakamura T, Sato T, et al. Laparoscopic surgery for locally advanced T4 colon cancer: the long-term outcomes and prognostic factors. Surg Today 2018;48:534-44. [Crossref] [PubMed]
- Tokunaga R, Xiu J, Johnston C, et al. Molecular Profiling of Appendiceal Adenocarcinoma and Comparison with Right-sided and Left-sided Colorectal Cancer. Clin Cancer Res 2019;25:3096-103. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Khalili M, Daniels L, Gleeson EM, et al. Pancreaticoduodenectomy outcomes for locally advanced right colon cancers: A systematic review. Surgery 2019;166:223-9. [Crossref] [PubMed]
- Li D, Si X, Wan T, et al. A pooled analysis of en bloc right hemicolectomy with pancreaticoduodenectomy for locally advanced right-sided colon cancer. Int J Colorectal Dis 2018;33:819-22. [Crossref] [PubMed]
- Huscher CGS, Lazzarin G. Coronary artery stent for securing pancreatico-jejunal anastomosis after PD: The "Huscher technique". Pancreatology 2022;22:1057-8. [Crossref] [PubMed]
- Meng H, Xu H, Wang X, et al. Total laparoscopic en bloc right hemicolectomy and pancreaticoduodenectomy with transvaginal specimen extraction for locally advanced right colon cancer: a case report. Gland Surg 2021;10:1780-5. [Crossref] [PubMed]
- Rajandram R, Khong TL, Aziz NA, et al. A narrative review: complete mesocolic excision in right-sided colonic cancer resections—present paradigm and future directions. Ann Laparosc Endosc Surg 2023;8:27. [Crossref]
- Guadagni S, Fiorentini G, Mambrini A, et al. Multidisciplinary palliation for unresectable recurrent rectal cancer: hypoxic pelvic perfusion with mitomycin C and oxaliplatin in patients progressing after systemic chemotherapy and radiotherapy, a retrospective cohort study. Oncotarget 2019;10:1-13. [Crossref] [PubMed]



