Camrelizumab combined with apatinib for unresectable, metastatic esophageal squamous cell carcinoma: a single-center, single-arm, prospective study
Highlight box
Key findings
• Camrelizumab combined with apatinib showed encouraging antitumor activity and controllable safety in locally advanced or advanced metastatic esophageal squamous cell carcinoma.
What is known and what is new?
• Antiangiogenic therapy can normalize tumor blood vessels in a short period, improve the infiltration of effector T cells and antagonize immunosuppressive signals to some extent, and enhance tumor immune activity.
• Apatinib combined with camrelizumab showed a more prolonged median overall survival (OS), which was much higher than the median OS of apatinib single-agent targeted therapy or camrelizumab single-agent immunotherapy in the second-line treatment of advanced metastatic esophageal squamous cell carcinoma.
What is the implication, and what should change now?
• Patients with advanced esophageal squamous cell carcinoma may benefit from an additional treatment option offered by this targeted combination immunotherapy model. At the same time, the impact of grade 3 and above adverse reactions on the quality of life patients cannot be ignored.
Introduction
Esophageal cancer (EC) is now one of the most common malignant tumors in the world. According to global cancer statistics in 2020, the number of new cases of EC reached 604,000, with 544,000 fatalities (1). China has a high incidence of EC, and although both this and mortality from the disease are decreasing, EC remains the primary malignant tumor threatening the health of residents. The primary histological type of EC in China is squamous cell carcinoma (SCC), which is closely related to diet and living habits, such as hot food, hot tea, drinking, and smoking. Despite all accessible treatments, which include chemotherapy, targeted therapy, local radiation therapy, and immunotherapy, the survival rate of these patients remains low, notably because of the late diagnosis and high recurrence rate, even in the case of local disease. In particular, among all ECs, esophageal squamous cell carcinoma (ESCC) has the worst prognosis, especially when metastatic disease occurs (2).
Targeted therapy and immunotherapy are increasingly used in cancer treatment. Targeted therapies related to EC mainly include the following categories: epidermal growth factor receptor (EGFR) pathway, vascular endothelial growth factor (VEGF), and anti-human epidermal growth factor receptor-2 (HER-2) treatment. Apatinib blocks downstream signal transduction and inhibits neovascularization in tumor tissues by highly selectively competing for the adenine adenosine triphosphate (ATP) binding site of VEGFR-2 (3). Recent studies have explored the efficacy and safety of apatinib in the posterior line treatment of EC. In a single-arm, open-label, phase II trial of patients with unresectable metastatic EC, administration of apatinib monotherapy (500 mg daily) resulted in overall survival (OS) (6.6 months) and progression-free survival (PFS) (4.6 months), with no intolerable adverse severe reactions (3). Another trial assessed the efficacy of apatinib combined with docetaxel versus docetaxel with S-1 as a second-line or third-line treatment for patients with advanced EC. The trial concluded that the combination of apatinib and docetaxel could be applied as a second- or third-line treatment for advanced EC, with an objective response rate (ORR) of 88.9%, a disease control rate (DCR) of 93.3%, and a significantly prolonged median PFS of 175 days (4). The superiority of apatinib combined with other treatment methods for EC has been confirmed in various studies (5,6), not only indicating a breakthrough in efficacy and prognosis but also showing synergistic and sensitizing effects.
Concurrently, clinical studies have established immunotherapy as achieving outstanding results in many tumor fields. Immunotherapy for ESCC mainly focuses on anti-programmed death 1 (PD-1), such as pembrolizumab, nivolumab, camrelizumab, and sintilimab, programmed death ligand 1 (PD-L1) drugs, adoptive cell therapy, and tumor vaccines. There are currently many advanced first-line immunotherapy studies, including ESCORT, a randomized, open-label, phase 3 study of patients with a histological or cytological diagnosis of advanced or metastatic ESCC. The results of this study have shown camrelizumab prolonged the median OS by >2 months, improved ORR by 13.8%, and reduced the risk of death by nearly 30% compared with chemotherapy (7). In June 2020, the indication for camrelizumab monotherapy for second-line treatment of advanced ESCC was officially approved, and immunotherapy has become the standard treatment for the disease. Although combination chemotherapy based on platinum and fluoropyrimidine/taxanes is commonly used as the first-line treatment of metastatic ESCC, the survival results are still disappointing, with a median OS of 8–10 months (7). In recent years, many randomized phase III clinical trials, such as Keynote-590 (8), ESCORT-1 (9), Checkmate 648 (10), ORIENT-15 (11), and JUPITER-06 (12), have shown encouraging results, providing more opportunities for the first-line treatment of advanced ESCC. Because ESCC has a high frequency of nonsynonymous mutations, radiosensitive mutations, and various antigen peptides, immunotherapy has unlimited potential in EC treatment. Therefore, it can be speculated that combination therapy based on immunotherapy supplemented by other antitumor therapy methods will become a new direction for EC treatment.
Although immunotherapy has succeeded in multiple types of tumors, prolonging overall patient survival, the ORR of immunotherapy has been unsatisfactory, which may be associated with the tumor immunosuppressive microenvironment. A study has produced extensive data showing antiangiogenic therapy can normalize tumor blood vessels in a short period, change the tumor microenvironment, improve the infiltration of effector T cells and antagonize immunosuppressive signals to some extent, and enhance tumor immune activity (13). Therefore, antiangiogenic therapy in combination with immunotherapy is promising as a novel therapeutic strategy to improve clinical outcomes. Phase 2 clinical trials of camrelizumab plus apatinib in various solid cancers have shown promising efficacy and manageable safety (5,14,15). Based on the results of these clinical studies and the efficacy of camrelizumab and apatinib in ESCC, we conducted a single-arm clinical trial to explore the efficacy and safety of combining the two as the first-line or second-line treatment of advanced ESCC. We present this article in accordance with the TREND reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-610/rc).
Methods
Study design
Our study was a single-arm, open-label trial conducted at the Affiliated Tumor Hospital of Xinjiang Medical University in China. The trial was registered in Chinese Clinical Trial Registry (No. ChiCTR2100046397). This clinical trial was carried out in compliance with the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice guidelines. The study was approved by China Ethics Committee of Registering Clinical Trials (No. ChiECRCT20210097). All patients voluntarily signed the informed consent.
Patient selection
The inclusion criteria were as follows: (I) patients were diagnosed as having locally advanced, locally recurrent, or metastatic ESCC by pathology. (II) Patients participated voluntarily, had good compliance, and could cooperate with the observation of the experiment. Before starting the research-related operations, all subjects signed an Inform Consent Form (ICF). (III) At least one measurable lesion was present, with a normal computed tomography (CT) or magnetic resonance imaging (MRI) scan lesion ≥20 mm or spiral CT ≥10 mm. (IV) The patient was aged 18–85 years, with good physical condition, and an Eastern Cooperative Oncology Group (ECOG) performance status ≤2. (V) The expected survival time was greater than three months. (VI) Laboratory inspection met the following standards: (i) routine blood test: white blood cell (WBC) ≥3.0×109/L, absolute neutrophil count (ANC) ≥1.5×109/L, platelet (PLT) ≥100×109/L, hemoglobin content (hemoglobin, HGB) ≥9.0 g/dL; (ii) liver function: aspartate transferase (AST) ≤2.5× ULN in subjects without liver metastasis, alanine aminotransferase (ALT) ≤2.5× ULN, liver transfer subjects with ALT and AST <5× ULN, serum total bilirubin (TBIL) ≤1.5× ULN (except for Gilbert syndrome TBIL <3.0 mg/dL), albumin (ALB) ≥3 g/dL; (iii) renal function: serum creatinine ≤1.5× ULN or creatinine clearance rate (CrCl) ≥40 mL/minute (using Cockcroft/Gault formula), urine protein (UPRO) is negative; (iv) coagulation function: international normalized ratio (INR) ≤1.5, activated partial thromboplastin time (APTT) ≤1.5× ULN, others: lipase ≤1.5× ULN; (v) if lipase >1.5× ULN and there was no clinical or imaging evidence of pancreatitis, the patient was included, amylase ≤1.5× ULN; (vi) if amylase >1.5× ULN and there was no clinical or imaging evidence of pancreatitis, the patient would be included; alkaline phosphatase (ALP) ≤2.5 ULN, subjects with bone metastases, ALP ≤5 ULN; (vii) male subjects and females of childbearing age must have undertaken contraception within 24 weeks after starting the first dose of the study drug to the last study drug (refer to protocol 6.6.2 for recommended contraceptive methods).
The exclusion criteria were as follows: (I) active brain metastasis or meningeal metastasis. Treated participants with brain metastases needed to meet the following standards before being included: (i) no MRI-proven progress ≥4 weeks after the end of treatment; (ii) complete treatment within ≥28 days before the first dose of the study drug. (II) Active tuberculosis (TB) or subjects with a history of active TB infection within 48 weeks before the screening, regardless of treatment. (III) The recent use of hormone therapy. (IV) Existing uncontrolled severe acute infections which were purulent and chronic infections in which the wounds were prolonged and unhealed. (V) Pre-existing severe cardiac illness, including congestive heart failure, uncontrollable high-risk arrhythmia, unstable angina, myocardial infarction, severe heart valve disease, and refractory hypertension; New York Heart Association grade ≥II heart functional insufficiency. (VI) The number of neutrophils in peripheral blood was lower than 1,500/mm3. (VII) Patients with unmanageable neurological or mental illnesses or disorders, as well as those with low compliance. (VIII) Pregnant and lactating women. (IX) Patients with other uncured malignant tumors.
Clinical treatment
All patients were given 200 mg of camrelizumab redissolved in 5 ml of sterile water for injection, diluted in 100 mL of 5% glucose injection or 0.9% sodium chloride injection, and delivered by intravenous drip for 30 min. At the same time, 250 mg of apatinib mesylate tablets were given orally once a day for three weeks as a treatment cycle, with at least two processes treated. The short-term efficacy and safety were evaluated. At least nine treatment cycles were completed, with ineffective subjects switched to other regimens.
In principle, patients with progressive disease (PD) that at least a 20% increase in the sum of the maximum diameter of the target lesions or the presence of new lesions ceased drug administration. However, if the investigator judged that taking medication might benefit the patient’s survival, they continued to take treatment until it was intolerable or PD recurred.
If patients presented with hematological treatment-related adverse events of grade 3 or worse, or nonhematological treatment-related adverse events of grade 2 or worse, camrelizumab or apatinib were interrupted until the hematological treatment-related adverse events resolved to grade 2 or better, or non-hematological treatment-related adverse events resolved to grade 1 or better. Camrelizumab or apatinib were permanently discontinued if dose interruption exceeded 8 or 4 weeks, respectively. Any patient needing to reduce the amount continued to receive the reduced dose treatment in the subsequent treatment cycle. The minimum dose was selected if the patient has multiple toxicities and the dose adjustment principles differed. If the dosage was reduced twice and needed to be reduced for a third time due to a toxic reaction, the treatment was stopped.
Outcomes and assessments
The primary endpoint was ORR, defined as the percentage of patients with complete response (CR) or partial response (PR) confirmed as the best overall response according to the investigator’s assessment. The study’s secondary endpoints included PFS (defined as the time from the start of treatment to the first disease advancement or death from any cause), OS (defined as the time from the initiation of therapy to death from any reason), DCR [defined as the proportion of patients who had a CR, PR, or stable disease (SD) as the best overall response], and treatment-related adverse reactions (TRAEs).
ORR was assessed every two cycles after treatment according to revised Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version1.1) (16). TRAEs were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. The survival status was followed up every month until death, consent withdrawal, or the study’s end. Patients with unknown or missing responses were treated as nonresponders.
Statistical analysis
All 29 patients were included in the practical statistical analysis. A waterfall chart presented each patient’s objective remission rate and disease development trend. The Kaplan-Meier method was used to estimate survival functions for OS and PFS, and median survival estimates were reported with 95% confidence intervals (CIs). LIFETEST procedure of SAS 9.4 was used to calculate OS and PFS and show the survival curves. The clinical data were classified by SPSS version 26.0.
Results
Demographics and baseline characteristics
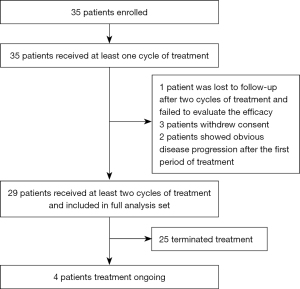
Between December 1, 2019 and July 31, 2022, 35 patients were enrolled according to the inclusion and exclusion criteria. Three patients refused to continue treatment, and disease in two patients showed noticeable progress after the first treatment period. One patient was lost to follow-up after two treatment cycles. By the end of the study, four (13.8%) patients were still receiving treatment, and 25 (86.2%) had terminated treatment, providing 29 patients for the statistical validity analysis (Figure 1).
Among the 29 patients, there were 18 males and 11 females, with a median age of 65 (range, 40–82 years). The type of disease was classified as local recurrence in nine (31.0%) patients, distant metastases in five (17.2%) patients, and simultaneous local recurrence and distant metastasis in 15 (51.7%) patients. Most patients were in good condition with ECOG performance status ranging from 0 to 1. Six (20.7%) patients had not received any treatment before enrollment, and the combination of camrelizumab and apatinib was used as the first-line treatment regimen. However, the remaining patients had received surgery, chemotherapy, radiotherapy, and concurrent chemoradiotherapy before enrollment. Fourteen patients completed at least six treatment cycles (18 cycles, n=1; 15 cycles, n=1; 11 cycles, n=1; ten cycles, n=2) (Table 1).
Table 1
| Baseline characteristics | Value (n=29), n (%) |
|---|---|
| Median age, years | |
| ≤65 | 16 (55.17) |
| >65 | 13 (44.83) |
| Sex | |
| Male | 18 (62.1) |
| Female | 11 (37.9) |
| ECOG performance status | |
| 0 | 20 (69.0) |
| 1 | 8 (27.6) |
| 2 | 1 (3.4) |
| Type of disease | |
| Local recurrence | 9 (31.0) |
| Distant metastases | 5 (17.2) |
| Local recurrence and distant metastases | 15 (51.7) |
| Location of metastases | |
| Lymph node | 25 (86.2) |
| Lung | 8 (27.6) |
| Liver | 5 (17.2) |
| Other | 10 (34.5) |
| Previous therapies | |
| Surgery | 4 (13.8) |
| Radiotherapy | 1 (3.4) |
| Chemotherapy | 9 (31.0) |
| Concurrent chemoradiotherapy | 13 (44.8) |
| None | 6 (20.7) |
| Treatment | |
| First-line | 9 (31.0) |
| Second-line | 17 (58.6) |
| Third-line | 3 (10.3) |
| Treatment cycle | |
| <6 cycles | 15 (51.7) |
| ≥6 cycles | 14 (48.3) |
ECOG, Eastern Cooperative Oncology Group.
Efficacy
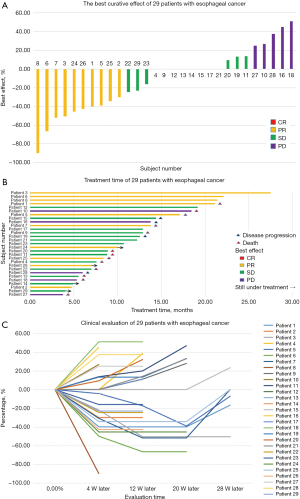
The median follow-up time after treatment was 10.7 months [interquartile range (IQR) 6.5–15.8 months]. The 29 patients included in the statistical analysis had good compliance, and none were lost to follow-up, while 16 (55.2%) died at the end of the study. By the end of the trial, no patient had achieved CR, 10 (34.5%) patients achieved PR, and 14 (48.3%) patients achieved SD, of which three patients had reduced target lesions but did not meet the PR standard. However, five patients showed significant progress in target lesions, including one with lung metastasis, one with bilateral clavicular lymph node metastasis, and the other three with enlarged original target lesions. The DCR of patients was as high as 82.8%, and an ORR of 34.5% was achieved (Table 2). Overall, 13 (44.8%) patients achieved shrinkage of their target lesions from the baseline (Figure 2A). Time receiving treatment in the analysis set, and disease development trend of each patient are shown in Figure 2B,2C respectively.
Table 2
| Variables | All patients (n=29) |
|---|---|
| Best overall response, n (%) | |
| Complete response | 0 |
| Partial response | 10 (34.5) |
| Stable disease | 14 (48.3) |
| Progressive disease | 5 (17.2) |
| ORR, n (%) | 10 (34.5) |
| DCR, n (%) | 24 (82.8) |
| Overall survival, months, median (95% CI) | 13.8 (11.2–16.2) |
| 6-month rate (95% CI) | 85.5% (65.7–94.3%) |
| 9-month rate (95% CI) | 80.9% (60.3–91.5%) |
| 12-month rate (95% CI) | 67.0% (43.8–82.4%) |
| Progression-free survival, months, median (95% CI) | 9.5 (7.0–13.6) |
ORR, objective response rate; DCR, disease control rate; CI, confidence interval.
By the end of the trial, 13 (29.5%) patients had died, and a total of 16 (36.4%) patients had PFS events (documented PD or death). The median OS was 13.8 months (95% CI: 11.2–16.2), and the estimated 6-, 9-, and 12-month OS rates were 85.5% (95% CI: 65.7–94.3%), 80.9% (95% CI: 60.3–91.5%), and 67.0% (95% CI: 43.8–82.4%), respectively (Figure 3A). The median PFS was 9.5 months (95% CI: 7.0–13.6; Figure 3B). Of those who died, one patient died of a sudden cerebral hemorrhage. Among patients with disease progression, seven patients had significantly thickened esophageal tube walls compared with the enrolled group, and one patient had obvious symptoms of dysphagia and anorexia. Recurrence patterns also included enlarged or new lymph nodes and distant metastasis, including two cases of brain metastasis, one case of lung metastasis, and two cases of liver metastasis.
Safety
The most common TRAEs are summarized in Table 3. All 29 patients were included in the safety analysis. One patient developed hyperbilirubinemia after completing four cycles of treatment, with both AST and ALT increased more than three times the expected value, leading to temporary discontinuation of treatment and use of liver protective drugs such as adenosine methionine and diammonium glycyrrhetinic acid. After two treatment cycles, one patient had a large red rash with pruritus on many body parts which may have been caused by camrelizumab, and the treatment was continued after complete remission through anti-allergic symptomatic treatment. However, the patient developed immune-related hepatitis after seven cycles of treatment, and the treatment was stopped because there was no noticeable improvement after glucocorticoid treatment (prednisone acetate, 1 mg/kg) for at least one month. After fifteen treatment cycles, one patient suffered from abdominal pain, diarrhea, and intermittent bloody stool. We consider the possibility of immunotherapy-related diarrhea is high but cannot exclude that of Crohn’s disease. Therefore, oral hormones, repair of the intestinal mucosa, anti-diarrhea, and hemostasis were given after stopping treatment. Two patients developed grade 3 reactive cutaneous capillary endothelial proliferation (CCEP). One patient terminated treatment because of liver dysfunction simultaneously, and the other temporarily interrupted camrelizumab and received local radiation therapy for simple lip telangiectasia.
Table 3
| Treatment-related adverse events | Any grade, n (%) | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) |
|---|---|---|---|---|
| White blood cell count decreased | 11 (37.9) | 6 (20.7) | 4 (13.8) | 1 (3.4) |
| Neutrophil count decreased | 9 (31.0) | 6 (20.7) | 3 (10.3) | 0 |
| Anemia | 12 (41.3) | 9 (31.0) | 3 (10.3) | 0 |
| Platelet count decreased | 8 (27.6) | 8 (27.6) | 0 | 0 |
| Hypoalbuminemia | 12 (41.3) | 11 (37.9) | 1 (3.4) | 0 |
| Aspartate aminotransferase increased | 10 (34.4) | 6 (20.7) | 3 (10.3) | 1 (3.4) |
| Alanine aminotransferase increased | 14 (48.2) | 7 (24.1) | 4 (13.8) | 3 (10.3) |
| Hypertension | 7 (24.0) | 1 (3.4) | 3 (10.3) | 3 (10.3) |
| Proteinuria | 5 (17.2) | 4 (13.8) | 1 (3.4) | 0 |
| Nausea | 1 (3.4) | 1 (3.4) | 0 | 0 |
| Anorexia | 5 (17.2) | 5 (17.2) | 0 | 0 |
| Thyroid function abnormal | 12 (41.4) | 10 (34.5) | 2 (6.9) | 0 |
| Reactive cutaneous capillary endothelial proliferation | 2 (6.9) | 0 | 0 | 2 (6.9) |
*, specific immune-related adverse reactions are detailed in the corresponding sections.
One patient developed proteinuria (3+) after five treatment cycles, stopped taking oral apatinib, and continued receiving single-agent immunotherapy with camrelizumab. Seven patients had hypertension symptoms of different degrees during the oral administration of apatinib, including three (10.3%) with grade 2 hypertension and three (10.3%) with grade 3 hypertension. All seven patients had no past history of hypertension, and three were complicated with diabetes. The blood pressure of these patients was controlled with antihypertensive drugs, and the trial treatment continued. After completing two treatment cycles, one patient refused to take apatinib orally because of his old age, previous chemotherapy, and repeated liver function abnormalities but he continued to receive single-agent immunotherapy with camrelizumab.
Grade 3 abnormal liver function was seen in three (10.3%) patients, ALT increased significantly, and grade 3 WBC count decreased in one (3.4%) patient. Abnormal thyroid function was observed in 12 (41.4%) patients, mainly hypothyroidism, and two patients had grade 2 hypothyroidism, which was treated with the oral hormone. No deaths occurred due to adverse events or other possible complications of the study treatment. Dose interruption of camrelizumab because of TRAEs occurred in 6.9% of patients, while treatment termination of camrelizumab due to TRAEs was also seen in 6.9% of patients. Treatment termination of apatinib due to TRAEs was seen in 6.9% of patients. Other adverse reactions including grade 3 proteinuria, hypertension, capillary hyperplasia, and decreased WBC count also occurred.
Discussion
EC is a severe threat to the life and health of Chinese people. For patients with locally advanced ESCC, preoperative neoadjuvant therapy can significantly improve the survival rate, but 70% still have metastasis or relapse six months after surgery (17). In China, 60–70% of EC patients have advanced lesions at the initial consultation. While radiotherapy and chemotherapy are the standard first-line treatment for progressive diseases, the treatment effect is often poor, and improving the 5-year survival rate of patients with EC, especially those with advanced disease, is imperative. Research on radiotherapy and chemotherapy for non-surgical EC patients has reached a bottleneck. However, treatment options for tumors have expanded in diversity in recent years due to the extensive study of tumor signal pathway-related targets and immunotherapy.
Immune checkpoint inhibitors have made incredible breakthroughs in the treatment of EC, and immunotherapy is now recommended by guidelines as the standard treatment scheme for first-line and second-line treatment of advanced EC. Because ESCC has biological characteristics such as high-frequency nonsynonymous mutation, radiosensitivity, and multiple antigenic peptides, immunotherapy has unlimited potential in EC treatment. However, although immunotherapy has achieved inevitable success in many types of tumors, which has prolonged the OS of patients, its response rate has been unsatisfactory. Some studies have confirmed that antiangiogenic therapy can facilitate the normalization of blood vessels and promote immune activation. At the same time, these drugs antagonize immunosuppressive signals and encourage immune tumor response (18-20). Therefore, the combination of antiangiogenic therapy and immunotherapy has attracted clinical attention.
The NCT03736863 study (21) was a single-arm, multicenter phase II trial exploring the second-line treatment of advanced ESCC with camrelizumab and apatinib. A total of 52 ESCC patients who progressed or could not tolerate first-line chemotherapy were enrolled. The results showed the ORR was 34.6%, the DCR was 78.8%, and the median PFS was 6.8 months. The NCT03603756 study (22) was a single-center phase II trial in China exploring the first-line treatment of advanced ESCC with camrelizumab, apatinib, and chemotherapy, in which 30 ESCC patients were enrolled, with an ORR of 80%, a DCR of 96.7%, and a median PFS of 6.85 months. While this study showed that combining camrelizumab and apatinib chemotherapy might become a new treatment option for locally advanced ESCC, a high incidence of TRAEs was seen, with a possible immune-related adverse reaction rate of 73.3%, of which 20% had grade 3–4 immune-related adverse reactions. Our study has also yielded encouraging results, showing an ORR of 34.5%, a DCR of 82.8%, and a median PFS of 9.5 months. Although none of the patients in our study achieved complete clinical remission, 44.8% had remission of the target lesions compared with the baseline level, and the disease remission rate of six (20.7%) patients reached more than 40%. At the same time, our results showed a more prolonged median OS, which was much higher than the median survival time of apatinib single-agent targeted therapy and camrelizumab single-agent immunotherapy in the second-line treatment of advanced ESCC. Therefore, we believe the superiority of apatinib combined with camrelizumab in treating EC has been confirmed in various studies in terms of efficacy and prognosis, and also shows synergistic and sensitizing effects. In treating locally advanced ESCC patients, apatinib combined with immunotherapy ensures the treatment intensity under the premise of controllable adverse reactions. This formation of complementary treatment methods provides a new idea for improving the OS of patients.
Although immunotherapy results are encouraging, its adverse reactions have gradually aroused the vigilance of clinicians. Immunotherapy-related adverse events (IRAEs) are a series of immune-mediated or immune-related adverse reactions caused by immune checkpoint inhibitors. The occurrence mechanism of IRAEs is unclear, and many characteristics different from the adverse reactions of traditional drugs are seen. A large meta-analysis published in 2019 found the incidence of full-level IRAEs was about 66%, and the incidence of grade 3 and above IRAEs was approximately 14.0% (23). In the safety analysis of the NCT03736863 study (21), adverse events occurred in 85% of 52 patients, with 79% of patients having treatment-related adverse events, of which 44% had grade 3 or 4, mainly elevated aspartate aminotransferase, elevated gamma-glutamyltransferase, or elevated ALT. In our trial, safety analysis revealed a similar incidence of grade 3 liver dysfunction. Even if there was no treatment-related death during the study, and most patients completed treatment on time after receiving timely symptomatic treatment, a small number of patients suspended or even ceased therapy due to adverse reactions. Therefore, we believe that although most clinical trial results show targeted combined immunotherapy mode is safe and effective, many immune-related adverse reactions can still be observed in actual clinical practice. Even though lethal IRAEs are unfamiliar, the impact of grade 3 and above adverse reactions on the quality of life of patients cannot be ignored. At the same time, although regulating tumor blood vessels through antiangiogenic therapy can improve the tumor immunosuppressive microenvironment, increase the number of infiltrating immune effector cells in the tumor, and provide immunostimulatory effects, whether this combined mode increases the toxic side effects of treatment needs to be explored.
This study has some limitations. First, this is a single-arm, single-center clinical study with a small sample size. Randomized, double-blind, multicenter phase III clinical trials to confirm the efficacy and safety of camrelizumab combined with apatinib are required. Second, as PD-L1 expression status was not explored, it was not possible to perform subgroup analysis according to the PD-L1 expression level.
Conclusions
Camrelizumab combined with apatinib shows encouraging antitumor activity and controllable safety in locally advanced or advanced metastatic ESCC. This targeted combined immunotherapy model provides a treatment option for patients with advanced disease. However, large-scale phase III clinical trials are needed to further verify its efficacy and safety.
Acknowledgments
We are very grateful to all the patients and their families participating in this clinical trial.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-610/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-610/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-610/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-610/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This clinical trial was performed in accordance with the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice guidelines. The study was approved by China Ethics Committee of Registering Clinical Trials (No. ChiECRCT20210097).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Petrillo A, Smyth EC. Immunotherapy for Squamous Esophageal Cancer: A Review. J Pers Med 2022;12:862. [Crossref] [PubMed]
- Yanwei L, Feng H, Ren P, et al. Safety and Efficacy of Apatinib Monotherapy for Unresectable, Metastatic Esophageal Cancer: A Single-Arm, Open-Label, Phase II Study. Oncologist 2020;25:e1464-72. [Crossref] [PubMed]
- Li J, Jia Y, Gao Y, et al. Clinical efficacy and survival analysis of apatinib combined with docetaxel in advanced esophageal cancer. Onco Targets Ther 2019;12:2577-83. [Crossref] [PubMed]
- Fan Y, Zhao J, Wang Q, et al. Camrelizumab Plus Apatinib in Extensive-Stage SCLC (PASSION): A Multicenter, Two-Stage, Phase 2 Trial. J Thorac Oncol 2021;16:299-309. [Crossref] [PubMed]
- Peng Z, Wei J, Wang F, et al. Camrelizumab Combined with Chemotherapy Followed by Camrelizumab plus Apatinib as First-line Therapy for Advanced Gastric or Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res 2021;27:3069-78. [Crossref] [PubMed]
- Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 2020;21:832-42. [Crossref] [PubMed]
- Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 2021;398:759-71. [Crossref] [PubMed]
- Luo H, Lu J, Bai Y, et al. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA 2021;326:916-25. [Crossref] [PubMed]
- Doki Y, Ajani JA, Kato K, et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med 2022;386:449-62. [Crossref] [PubMed]
- Shen L, Lu Z, Wang J, et al. LBA52 Sintilimab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced or metastatic esophageal squamous cell cancer: first results of the phase III ORIENT-15 study. Ann Oncol 2021;32:S1330. [Crossref]
- Xu R, Wang F, Cui C, et al. 1373MO JUPITER-06: A randomized, double-blind, phase III study of toripalimab versus placebo in combination with first-line chemotherapy for treatment-naive advanced or metastatic esophageal squamous cell carcinoma (ESCC). Ann Oncol 2021;32:S1041. [Crossref]
- Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol 2018;15:310-24. [Crossref] [PubMed]
- Zhou C, Wang Y, Zhao J, et al. Efficacy and Biomarker Analysis of Camrelizumab in Combination with Apatinib in Patients with Advanced Nonsquamous NSCLC Previously Treated with Chemotherapy. Clin Cancer Res 2021;27:1296-304. [Crossref] [PubMed]
- Xu J, Shen J, Gu S, et al. Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-label, Phase II Trial. Clin Cancer Res 2021;27:1003-11. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 2018;36:2796-803. [Crossref] [PubMed]
- Song Y, Fu Y, Xie Q, et al. Anti-angiogenic Agents in Combination With Immune Checkpoint Inhibitors: A Promising Strategy for Cancer Treatment. Front Immunol 2020;11:1956. [Crossref] [PubMed]
- Zheng B, Zhou C, Qu G, et al. VEGFR2 Promotes Metastasis and PD-L2 Expression of Human Osteosarcoma Cells by Activating the STAT3 and RhoA-ROCK-LIMK2 Pathways. Front Oncol 2020;10:543562. [Crossref] [PubMed]
- Raybould AL, Sanoff H. Combination Antiangiogenic and Immunotherapy for Advanced Hepatocellular Carcinoma: Evidence to Date. J Hepatocell Carcinoma 2020;7:133-42. [Crossref] [PubMed]
- Meng X, Wu T, Hong Y, et al. Camrelizumab plus apatinib as second-line treatment for advanced oesophageal squamous cell carcinoma (CAP 02): a single-arm, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2022;7:245-53. [Crossref] [PubMed]
- Zhang B, Qi L, Wang X, et al. Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first-line treatment of advanced esophageal squamous cell carcinoma. Cancer Commun (Lond) 2020;40:711-20. [Crossref] [PubMed]
- Wang Y, Zhou S, Yang F, et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol 2019;5:1008-19. [Crossref] [PubMed]
(English Language Editor: B. Draper)