An unexpected case of large cell neuroendocrine carcinoma of the colon: a case report
Highlight box
Key findings
• Combination of North American Neuroendocrine Tumor Society and European Neuroendocrine Tumor Society consensus treatment guidelines for the treatment of large cell neuroendocrine carcinoma (LCNEC) of the colon.
What is known and what is new?
• LCNECs of the colon are rare and highly aggressive cancers with high rates of mortality at both 1- and 5- year reference points.
• Treatment modalities are still being standardized.
• A potential standardized treatment modality via the combination of consensus guidelines.
What is the implication, and what should change now?
• As it stands, the malignancy is in remission and the patient is successfully being treated with minimal side effects. To that end, our case presents a possible standardized treatment modality.
Introduction
Neuroendocrine carcinomas (NECs) are rare oncological manifestations comprising less than 2% of total colon and rectal cancers; additionally, large cell NECs (LCNECs), as with our patient, constitute approximately 0.25% of colorectal cancers (1,2). Due to the highly invasive nature of LCNECs, prognosis is unfavorable especially as the cancer is metastatic at the point of diagnosis (1). Regarding metastasis at the point of diagnosis, one study reported a “silent” version of the cancer, which only presented as anemia despite being stage IV with additional metastasis to the liver, lung, bone, and lymph nodes (3). Notably, LCNECs can resemble poorly differentiated adenocarcinomas; thus, it is critical to examine neuroendocrine markers to avoid diagnosis prolongation considering the poor prognosis and high metastatic potential (4). Histologically, high mitotic rates, large polygonal cells with coarse chromatin, as well as forming nesting, organoid, trabecular, rosette, and palisading patterns (5). In 2010, World Health Organization (WHO) classified neuroendocrine tumors into categories based on grade as indicated by the Ki-67 index (4). Per the index, G1 (Ki-67 index ≤2%) and G2 tumors (Ki-67 index 3–20%) are well-differentiated while G3 (Ki-67 index >20%) tumors are poorly differentiated (6). Guidelines regarding the specific management modalities of G3 tumors are based on level of metastasis, but consensus indicates surgery as well as adjuvant chemotherapeutic approaches (7-9).
We report a case of a 69-year-old male who presented with diffuse pain in the left upper abdomen and a positive at-home screening test who was diagnosed with LCNEC via biopsy of the proximal ascending colon. We present this article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-542/rc).
Case presentation
We report a case of a 69-year-old male with a positive at-home cancer screening test. During the initial patient visit, he reported non-severe pain in the left epigastric region, and denied hematochezia, melena, constipation, and diarrhea. The patient denied any changes in medical history since previous visits with physicians. The patient has a past medical history of hypertension and colonic polyps. Both parents have a history of malignant neoplasms. Our patient’s mother had breast cancer and father had bladder cancer. Regarding social history, the patient denied any prior alcohol, tobacco, and drug use. The patient is self-employed and has no occupational exposure to toxic chemicals.
As a result of the positive screening test, a colonoscopy was performed, and biopsies were conducted along the proximal to distal length of the colon. Cells collected from a sample obtained within the proximal ascending colon were identified to be malignant. These cells demonstrated positively for pankeratin, synaptophysin, and dim CDX2. Additionally, there were negative for CK7, CK20, chromogranin, PSAP, TTF-1, and GATA3. The Ki-67 proliferative index of this sample was approximately 75%. Further, within the distal ascending colon, biopsy indicated a separate, detached fragment of NEC that was morphologically similar to the biopsy obtained from the proximal ascending colon. It also stained positively for synaptophysin and negatively for CK7, CK20, and chromogranin. The Ki-67 proliferative index in this biopsy sample was approximately 70%. Pathology further indicated the distal ascending colon biopsy was a detached fragment from the tumor within the proximal ascending colon biopsy and not a secondary site of the tumor.
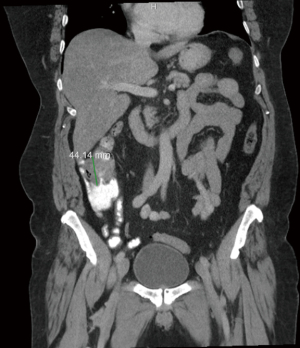
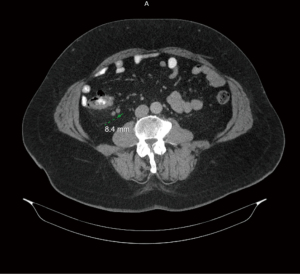
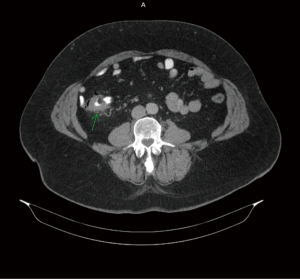
Figures 1-3 contain imaging taken prior to the right hemicolectomy, which are multiple contiguous axial computed tomography (CT) images. As illuminated in gastrointestinal (GI) CT 1, there is a 4.4 cm mass within the proximal ascending colon, which is the assumed colonic malignancy. The arrow in CT 2 indicates prominent pericolic lymph nodes that are potential representations of metastasis. More of these can be seen in CT 3.
As per treatment guidelines, our patient was scheduled for a right hemicolectomy with ileocolic anastomosis. The procedure robotic surgery involved optical entry into the right upper quadrant (RUQ) and subsequent robotic working ports into the left upper quadrant (LUQ) utilizing a transversus abdominis plane (TAP) block with Exparel and general anesthesia. During the operation, the mesentery was divided into the proximal transverse colon and dissected superiorly from inferior to the hepatic flexure. A second dissection plane was established via approach from inferior to the ileocolic vessel communicating to the first dissection plane. The mesentery of the small bowel was dissected by approximately 15 cm. Once removed, the segment of the right ascending colon was sent to pathology for final evaluation.
The final report indicated that the LCNEC was a unifocal tumor approximately 3.6 cm × 3.1 cm × 1.1 cm in size. It invaded transmurally through the muscularis propria extending into the serosal fat, which was all resected during the operation. Histologically, the tumor had a mitotic rate of 40/10 high-power field (hpf) with a Ki-67 index of 70% in agreement with previously biopsied samples. Additionally, the 4/18 regional lymph nodes contained metastatic LCNEC with two additional larger lymph nodes showing focal extranodal extension of the tumor into the adjacent fat. All six identified lymph nodes were resected during the procedure. Final diagnosis by pathology indicated a poorly differentiated stage IIIA (T3, N1, M0) LCNEC of the right ascending colon with Ki-67 index at 70%.
Two months post-operatively, the patient was seen for follow-up imaging as seen below. During this period, the patient reports mild diarrhea but is otherwise asymptomatic. The patient case was presented during GI conferencing wherein pharmacological treatment interventions were agreed upon. The patient’s treatment plan would consist of six cycles of carboplatin and etoposide with routine follow-up with labs and imaging. Post-operative management of LCNECs may also include cisplatin as an alternative to carboplatin (8). The GI conference committee considered cisplatin instead of carboplatin, but proposed carboplatin in light of it being less nephrotoxic with less neurological sequelae. For this patient, adjuvant radiation was not considered given current guidelines and the successful hemicolectomy.
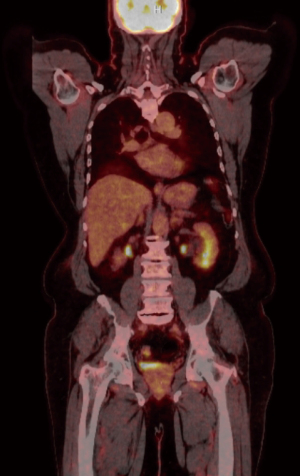
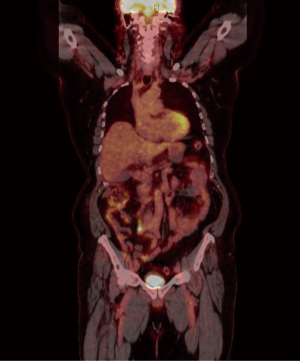
Figures 4,5 highlight the changes associated with the patient’s right hemicolectomy. There was no indication of fludeoxyglucose (FDG) avid lymph nodes. The anterior right abdominal wall demonstrates a standardized uptake value (SUV) maximum of 5.7.
The patient, to date, has completed six full rounds of carboplatin and etoposide. Physical examination and reactive oxygen species (ROS) findings were unremarkable outside of changes to bowel habits associated with surgery. The patient has made a full return to all pre-disease performance without restriction as indicated by the pulmonary embolism (PE) performance scale: Eastern Cooperative Oncology Group (ECOG) performance with a grade scale score of 0.
Table 1 indicates the progression of laboratory values across the first 28 days after treatment initiation.
Table 1
| Date | WBC, ×103/μL | HGB, g/dL | HCT, % | Platelets, ×103/μL |
|---|---|---|---|---|
| Day 0 | 7.95 | 12.2 | 39.3 | 268 |
| Day 7 | 7.18 | 12.4 | 39.4 | 282 |
| Day 18 | 7.2 | 11.2 | 35.4 | 284 |
| Day 28 | 3.98 | 11 | 34.9 | 321 |
The progression of laboratory values across the first 28 days after treatment initiation. WBC, white blood cell; HGB, hemoglobin; HCT, hematocrit.
The patient developed anemia secondary to both cancer and chemotherapy, which was being monitored and assessed via routine laboratory analysis. After the second round of chemotherapy, the anemia was improved nor were there any findings associated with vitamin or mineral deficiencies. However, on complete blood count (CBC) with differential after the second dose, showed marked elevations to both lymphocyte and monocyte percentages above normal range with an associated reduction in neutrophil percentages. Finally, there was also development of a fungal dermatological infection in the axillary region, which is being treated with an antifungal agent. Both the CBC abnormalities and the fungal infection were resolved prior to the third dose. Doses three through six were unremarkable regarding lab or examination findings. Six months after starting chemotherapy, the patient received a new positron emission tomography (PET) scan, which showed no evidence of hypermetabolic disease with stable examination findings. Further, carcinoembryonic antigen (CEA) at this time was found to be 1.9 without patient complaints.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made. Discussion with patient to obtain consent have been lost to follow-up as of this point in time.
Discussion
LCNECs are prognostically poor cancers due to both the aggressive nature of the malignancy itself and the high chance of metastasis at the point of diagnosis with a 1-year survival rate at approximately 10% and a median survival time of 5 to 11 months (5,7). There are not formally established universal treatment modalities for LCNECs given the highly invasive nature of the cancer (6-9). The relative 5-year survival was 16.3% across all staging of NECs and 57.4%, 56.4%, 26.3%, and 3.0% at stages I, II, III, and IV respectively (9). European Neuroendocrine Tumor Society (ENETS) and North American Neuroendocrine Tumor Society (NANETS) consensus guidelines follow a similar algorithm to initial evaluation and staging of the NEC (9,10). According to both guidelines, colonoscopy, biopsy, and imaging should be conducted (9,10). NANETS recommends colonoscopy, CT, magnetic resonance imaging (MRI), and octreotide scintigraphy (10). Whereas ENETS recommends colonoscopy, CT, MRI, and FDG-PET be performed for initial imaging (9). In our patient, we performed all of these tests except for octreotide scintigraphy. Both NANETS and ENETS suggested evaluation of heart, liver, and kidney function as well as pathology to look for synaptophysin, chromogranin A, and Ki-67 (9,10). Consensus guidelines regarding treatment suggest surgical resection of the malignancy with routine follow-up and monitoring via FDG uptake on PET scan as well as colonoscopy on a 1-to-2-year basis (11). In patients with non-small cell NECs, surgery provided increased overall survival with a median of 21 months as opposed to those who did not undergo surgery with a median of 6 months (P<0.0001) (7). Additionally, management post-operatively may include adjuvant chemotherapy with either cisplatin or carboplatin and etoposide for 4–6 cycles (8). Regarding follow-up, the guidelines differ slightly, ENETS recommends 3-month follow-up while NANETS recommends 3 to 6 months after resection with a shift to every 6 to 12 months for at least 7 years (9,10).
Regarding our patient, the incidental finding of LCNEC was unexpected as our patient only presented with abdominal pain at the initial visit without any of the more traditional or common symptoms (12). Our patient is currently 5 months post-operative at the time of writing with no signs of malignancy and a positive return to pre-disease state performance. Initial imaging prior to surgery indicated a 4.4 cm semicircular mass within the proximal ascending colon, which was subsequently resected via right hemicolectomy, which was ultimately determined to be 3.6 cm × 3.1 cm × 1.1 cm in size after surgical resection. Additionally, there were mild prominent pericolic lymph nodes noted, which were potentially concerning for metastasis. These lymph nodes were resected during the hemicolectomy procedure. Follow-up PET scan post-operatively imaging showed no signs of malignant growth or FDG uptake via the pericolic lymph nodes. Our treatment and management of the primary LCNEC malignancy consisted of a combination of the aforementioned treatment modalities including both surgical resection and adjuvant chemotherapy (8-10).
Conclusions
In relation to other colon and rectal cancers, LCNECs comprise less than 1% (1,2). These tumors are highly aggressive leading to poor prognostic outcomes with high mortality rates on both 1- and 5-year survival scales. For our patient, the novelty in his treatment and current outcomes are related to the finding of LCNEC incidentally and early prior to the rapid metastasis to additional organs indicated by other reports (3). In addition, previous reports also indicated a need for further research and data discussing the standardization of treatment for patients diagnosed with LCNECs (5). In lieu of this, our study highlights a potential standardized treatment modality via the combination of both ENETS and NANETS consensus guidelines with a minor change to replace cisplatin with carboplatin further emphasizing the novelty in our report (8,9). It is important to note, however, that our case report is only acknowledging a short-term perspective of 5 months, and as such, longer-term oncological outcomes are unknown.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-542/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-542/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-542/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made. Discussion with patient to obtain consent have been lost to follow-up as of this point in time.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bernick PE, Klimstra DS, Shia J, et al. Neuroendocrine carcinomas of the colon and rectum. Dis Colon Rectum 2004;47:163-9. [Crossref] [PubMed]
- Vilar E, Salazar R, Pérez-García J, et al. Chemotherapy and role of the proliferation marker Ki-67 in digestive neuroendocrine tumors. Endocr Relat Cancer 2007;14:221-32. [Crossref] [PubMed]
- Baek HS, Kim SW, Lee ST, et al. Silent advanced large cell neuroendocrine carcinoma with synchronous adenocarcinoma of the colon: A case report. World J Gastrointest Oncol 2022;14:2266-72. [Crossref] [PubMed]
- Ilett EE, Langer SW, Olsen IH, et al. Neuroendocrine Carcinomas of the Gastroenteropancreatic System: A Comprehensive Review. Diagnostics (Basel) 2015;5:119-76. [Crossref] [PubMed]
- Khanna V, Reddy T, Nagar T, et al. Metastatic Large Cell Neuroendocrine Carcinoma of the Colon: A Case Report. Cureus 2022;14:e26075. [Crossref] [PubMed]
- Jernman J, Välimäki MJ, Louhimo J, et al. The novel WHO 2010 classification for gastrointestinal neuroendocrine tumours correlates well with the metastatic potential of rectal neuroendocrine tumours. Neuroendocrinology 2012;95:317-24. [Crossref] [PubMed]
- Shafqat H, Ali S, Salhab M, et al. Survival of patients with neuroendocrine carcinoma of the colon and rectum: a population-based analysis. Dis Colon Rectum 2015;58:294-303. [Crossref] [PubMed]
- Strosberg JR, Coppola D, Klimstra DS, et al. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas 2010;39:799-800. [Crossref] [PubMed]
- Garcia-Carbonero R, Sorbye H, Baudin E, et al. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology 2016;103:186-94. [Crossref] [PubMed]
- North American Neuroendocrine Tumor Society. 2022 edition NANETS guidelines. 2022. Available online: https://nanets.net/images/guidelines/21513_NANETS_2022_Guidelines_Compendium.pdf
- Knigge U, Capdevila J, Bartsch DK, et al. ENETS Consensus Recommendations for the Standards of Care in Neuroendocrine Neoplasms: Follow-Up and Documentation. Neuroendocrinology 2017;105:310-9. [Crossref] [PubMed]
- Vilallonga R, Espín Basany E, López Cano M, et al. Neuroendocrine carcinomas of the colon and rectum. A unit's experience over six years. Rev Esp Enferm Dig 2008;100:11-6. [Crossref] [PubMed]