Repeat cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: review of indications and outcomes
Introduction
Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) is currently offered in many centers across the United States, treating peritoneal surface disease predominantly from appendiceal, colorectal, ovarian and mesothelioma primaries.
Patient selection for CRS/HIPEC is based on the type of primary, volume and topography of peritoneal disease as well as age, functional status and comorbidities (1). Despite the efficacy of CRS/HIPEC in treating peritoneal surface disease, the majority of patients will eventually develop recurrent disease (2-4). Recurrence following CRS/HIPEC usually occurs in an isolated intra-abdominal location in 31-57% of patients (4-7). Although a subset of patients with recurrence may remain asymptomatic for prolonged intervals, the majority of patients eventually die from intestinal obstruction and malnutrition (8,9). Given this pattern, patients with late peritoneal recurrence without a concomitant extra-abdominal component, can be evaluated for repeat CRS/HIPEC. These patients in addition, should have preserved nutritional and ECOG status. Repeat CRS/HIPEC is associated with similar morbidity and mortality compared to the initial operation. In our experience only 7% of patients receive repeat CRS/HIPEC, with the ability to achieve a complete macroscopic cytoreduction and the time interval between the two procedures being the two major factors determining patient selection and survival (10) (Tables 1,2).
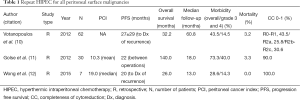
Full table
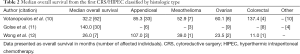
Full table
The primary aim of this review is to present the available outcomes data on repeat CRS/HIPEC procedures.
Appendiceal primaries
Pseudomyxoma peritonei (PMP) from appendiceal primaries include tumors with a wide spectrum of biologic behavior that ranges from chronic indolent disease to rapid progression with distant metastasis. The two tier Wake Forest Classification described by Bradley et al. (13,14) stratifies patients with mucinous carcinoma peritonei into two groups: low and high grade which combines the 3 groups described by Ronnett et al. in 1995 (15). More specifically, low-grade tumors include those with disseminated peritoneal adenomucinosis (DPAM), well-differentiated mucinous carcinomatosis, peritoneal mucinous carcinomatosis with intermediate or discordant features (PMCA I/D) and well-differentiated variants of mucinous adenocarcinoma or low-grade appendiceal mucinous neoplasms. High-grade tumors include moderate or poorly-differentiated adenocarcinoma, PMCA, and cases with signet-ring cell components. Categorization of appendiceal primaries as either low- or high-grade provides the prognostically relevant pathological grade and utilizes terminology that is appropriate to the clinical context.
Over the past few decades, the standard of care has changed from debulking only, to cytoreduction followed by HIPEC. In one of the earliest studies of patients with PMP, Gough et al. at the Mayo Clinic reported surgical outcomes of 56 patients with predominantly appendiceal and ovarian primaries (16). HIPEC was administered in 13% of the cohort. With a median follow up of 12 years, disease recurrence was noted in 76% with 50% of the recurrences noted within 2.5 years from initial surgery. Repeat CRS was performed in 71% of patients that suffered the recurrence (11% of those with HIPEC), but only 20% had a complete cytoreduction. Even though CRS with HIPEC prolonged recurrence free survival, these historic data include patients from a period when pathological classification of PMP was not well understood, criteria for treatment were not well defined, and detection of recurrence was based on follow up physical exam rather than modern imaging.
Esquivel and Sugarbaker published one of the first series of patients undergoing second look after initial CRS/HIPEC. Of the initial 321 patients, 98 (30.5%) underwent a second look operation for multiple indications, of which 79 patients underwent a repeat CRS/HIPEC (17). The overall 5-year survival of these patients was 73.6% compared to 68% for the patients that did not undergo reoperation. Small bowel involvement was associated with a 28% 5-year survival despite second look CRS/HIPEC. Completeness of cytoreduction was associated with improved long-term survival (84% 5-year survival). The authors emphasized the importance of a staged approach in patients with high PCI (peritoneal cancer index) and wide dissemination. They suggested complete cancer removal from targeted anatomic sites with planned second-look for the rest of the involved areas. This staged approach offers the chance to assess the response of the cytoreduced areas. Durable response with a decrease in the PCI of the treated areas is a favorable indicator for survival. They concluded that repeat CRS in patients with appendiceal primaries is feasible with good overall long-term survival because of infrequent metastasis outside the peritoneal cavity, compressive rather than invasive behavior of the recurrent disease, relative sparing of small bowel and a good response to intraperitoneal chemotherapy.
In a more recent study from the same center, Yan et al. analyzed a cohort of 402 patients with PMP from low and high-grade appendiceal primaries treated with CRS and HIPEC (18). Of those, 111 (28%) were found to have disease progression on follow-up and 98 had repeat CRS-HIPEC with significantly improved survival (75% at 5 years) compared to those that did not undergo a repeat procedure. Only incomplete cytoreduction was identified as an independent predictor of reduced survival on multivariate analysis. Fifty three of the 98 patients had disease progression, of which 26 underwent a third CRS with or without HIPEC. Overall survival in patients that underwent iterative CRS was better than those that did not undergo a repeat procedure. PMCA was found to be associated with reduced recurrence-free and overall survival though the 10-year survival of complete CRS was reported to be 75%, significantly higher than the 10-20% reported in historic controls.
In an attempt to further define the viability of repeated attempts at CRS/HIPEC, the same group studied 45 patients with appendiceal cancer and carcinomatosis that underwent 3 or more operative procedures. The 5- and 10-year survival rates reported were: 60% and 48% for 3, 78% and 36% for 4 and 100% and 80% for 5 or more interventions, respectively (19). Complete cytoreduction, especially at the subsequent procedures was associated with improvement in survival. Tumor histology, indication for repeat surgery and nodal status did not have a significant impact on survival regardless of the number of surgical interventions. An interesting finding in this study was the change in histologic type in subsequent procedures in 21 (47%) patients. In 14 patients, a more aggressive histologic type was noted whereas the remaining had less aggressive pathologic type noted on repeat CRS; however, survival was not statistically impacted by change in tumor histology. The authors describe several possible etiologies for this change including incorrect initial diagnosis, dedifferentiation, sampling error and direct cytotoxic effects from intraperitoneal chemotherapy resulting in less aggressive tumor biology.
Miner et al. published their 22-year cohort with PMP from the Memorial Sloan Kettering Cancer Center (20). They identified 97 patients, 52% with low grade and 48% with high grade appendiceal adenocarcinoma who underwent 202 operations. 55% of patients underwent a complete CRS. About 91% of these patients recurred with a median disease-free interval of 24 months. About 70% of patients had repeat CRS: 39% had 2, 21% had 3, 7% had 4 and 3% had 5 or more operations. Tumor grade and completeness of cytoreduction were independently associated with prolonged survival. Median survival for low grade tumors was 12.8 vs. 4 years for high grade tumors. Perioperative mortality was 4%, morbidity rate was based on the extent of surgery score and was highest at 38% for extensive CRS involving >5 regions.
Sardi et al. reported 54% and 34% 5-year survival rate after the first and second CRS/HIPEC respectively, in a cohort of 22 patients with peritoneal carcinomatosis of appendiceal origin who underwent repeat HIPEC (21). With regard to specific histopathology, PMCA (high-grade) patients had a median survival of 53 and 29.9 months after the first and second CRS/HIPEC respectively whereas DPAM (low-grade) patients had 90% 5-year survival after the first CRS/HIPEC and 100% after the second. Major complication rate was 43% whereas there was no 30-day or in-hospital mortality. PCI rates were not significantly different between the two groups with 65% of patients in both groups having PCI ≥20.
In a study published from our center for repeat cytoreduction, 33/62 patients who underwent repeat CRS/HIPEC had an appendiceal primary (10). Median overall survival for this subgroup after the second CRS/HIPEC was 52.1 months. Resection status for the second CRS and time interval between the two procedures were associated with survival in multivariate analysis. Overall morbidity, including minor and major, was 43.5% and mortality was 3.2%.
Repeat CRS/HIPEC for appendiceal primaries appears to be a reasonable option for patients with limited, isolated peritoneal recurrence with meaningful long-term outcomes and acceptable morbidity and mortality in experienced centers (Table 3).
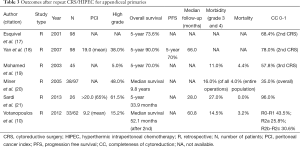
Full table
Colorectal primary
Colorectal cancer represents the third most common type of cancer in the developed world with common areas of metastatic disease including the lymph nodes, liver, lungs and the peritoneum (22). Historically, 5-10% of patients with colorectal carcinoma present with synchronous metastases of the peritoneum and 20-50% present with metachronous disease (23,24). Prospective randomized clinical trials, case control studies and meta-analyses have demonstrated the benefit of CRS/HIPEC in the setting of peritoneal carcinomatosis from colorectal cancer for selected patients, with 5-year survival between 17-51% in selected patients (2,25-28). A Consensus statement published in 2006 recommended the use of CRS/HIPEC for peritoneal carcinomatosis of colorectal origin and discussed variables that are commonly associated with a complete cytoreduction, which has been shown to be a predictive factor of survival. These factors included ECOG performance status, no evidence of extra-abdominal disease, up to three small resectable hepatic metastases, absence of several segmental sites of malignant small bowel obstruction, small volume disease in the gastro-hepatic ligament and no evidence of biliary/ureteral/small bowel obstruction in more than one site (29). However, even with complete cytoreduction, disease recurrence at a median interval of 6-12 months occurs in 48-70% of patients, with 31-57% of them presenting with isolated peritoneal disease (4,7,30). Therefore, selected patients will be considered for repeat cytoreduction. Given that there are no randomized trials addressing the role of repeat CRS/HIPEC in colorectal cancer, most of the data are derived from retrospective studies.
In one of the earliest studies, 18 patients with recurrent colorectal cancer who underwent repeat CRS/HIPEC were analyzed (31). Completeness of cytoreduction both at the first and the repeat procedure were found to positively correlate with improved survival. Following a repeat CRS/HIPEC procedure, with a median survival of 20 months, an improved survival at 2 years was noted in patients that had a PCI score of 12 or less.
In a large retrospective multicenter study that included 506 patients from 28 institutions, an overall recurrence rate of 64.2% and peritoneal recurrence rate of 41.9% was described for patients undergoing complete cytoreduction (32). Twenty six patients underwent a second procedure with significantly improved median survival of 57.6 months. The completeness of the cytoreduction score, use of adjuvant chemotherapy and a second procedure performed were significant predictors for improved survival while the reported mortality and morbidity rates were 4% and 22.9%, respectively. The use of neoadjuvant chemotherapy, lymph node involvement, presence of liver metastasis, and poor histologic differentiation were negative independent prognostic indicators. The median disease-free survival was 13 months leading the authors to recommend a second look procedure for patients who remained without disease at 1 year from their initial operation.
In another study from France, 43 patients with peritoneal carcinomatosis with or without liver metastases from colorectal cancer were studied with 26% undergoing repeat CRS/HIPEC (33). Of note, 26% of patients had liver resection prior to, at the same time or after the initial CRS/HIPEC. A total of 70% of patients underwent complete CRS after the second procedure compared to a 50% rate of complete CRS achieved during the first operation. Median survival after repeat CRS/HIPEC was 38.4 months and 4-year survival was reported at 44%. There was no survival difference between patients undergoing CRS/HIPEC for peritoneal carcinomatosis alone or those undergoing concomitant liver resection (median survival 35.3 vs. 36 months). Mortality rate was 2.3% and reported morbidity rate was 39%. Similar to other reports, completeness of the cytoreduction was found to be a significant predictor of survival on multivariate analysis. The authors concluded that there was a definitive role for repeat CRS/HIPEC in selected patients with peritoneal carcinomatosis for colorectal cancer.
In their study of 70 patients undergoing CRS/HIPEC for colorectal carcinoma, Bijelic et al. documented recurrences in 49 patients, 36.7% of whom had isolated localized peritoneal disease (34). Twenty six patients underwent a second CRS/HIPEC with a median survival that was significantly longer than that of patients who did not have a second operation (39 vs. 20 months). Three patients were still alive without evidence of disease 5 years after diagnosis of recurrence. The authors postulate that localized isolated intraperitoneal recurrences could be considered a form of surgical failure, likely secondary to inability of intraperitoneal chemotherapy to eradicate tumor cells being trapped within scar tissue. They cautioned against repeat CRS/HIPEC in patients with diffuse intra-abdominal recurrence, as these patients had much poorer long-term survival. Given that the extent of disease and ability to achieve a complete cytoreduction are major factors associated with overall survival, a recent study similarly demonstrated no additional survival benefit from CRS/HIPEC over palliative surgery in patients with PCI higher than 17 (35).
In a recent review, Williams et al. attempted to clarify the role of CRS/HIPEC for tumor recurrence by presenting data from their own institution and that from other reported series in the literature (36). The study included 18 patients from the authors’ institution with reported median survival of 59 months from first and 22.6 months from second CRS comparing favorably to the other reported series between 20 and 57.6 months. The observed major complication rate was 16.7% and there was no postoperative mortality, results identical to those presented from different studies in the literature. However given the small number of patients and lack of randomized trials for patients undergoing repeat CRS/HIPEC, they concluded that patient selection should be tailored based on a multidisciplinary approach.
In one of the largest series, Braam et al. studied 287 patients undergoing CRS/HIPEC for colorectal primary, of which 132 (46%) were diagnosed with recurrent disease after a median disease-free interval of 11.4 months (37). 43% of these were isolated locoregional recurrences. Of the 132 patients, 32 with recurrences (24%) were treated surgically with curative intent, which increased median overall survival from 12 to 43 months, compared to palliative treatment. Lymph node status and number of regions involved at initial CRS were the strongest predictors of long-term outcome.
Repeat CRS/HIPEC for colorectal primary, therefore appears to be a feasible practice in carefully selected patients, with completeness of cytoreduction and low tumor burden being predominant predictors of improved survival (Table 4).
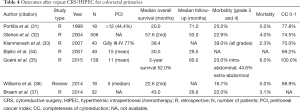
Full table
Ovarian cancer
Epithelial ovarian cancer (EOC) is the commonest cause of gynecologic cancer deaths in the western world and the fifth most common cause of cancer deaths in U.S. women, with over 14,000 deaths expected in 2015 (38,39). Most patients present with metastatic disease and a majority of patients with stage III ovarian cancer are treated with upfront CRS and systemic platinum based chemotherapy, with a high initial response rate (40). Despite this, 60% of patients with a good response will recur within 5 years while another 20% of patients are inherently resistant to platinum based regimens (41,42). No current standard of care has been defined for recurrent patients.
In accordance with an early Cochrane database review (43), many patients have been historically treated with systemic chemotherapy alone and a few select patients are offered repeat CRS. In two separate meta-analyses, Bristow et al demonstrated the importance of complete cytoreduction in improving overall survival; referral to a specialized center and completeness of cytoreduction being the most significant determinants of improved long-term survival (40,44). In an updated analysis of the Cochrane database that included 1,194 patients from 9 studies, complete cytoreduction was found to be associated with a significant improvement in overall survival for platinum-sensitive recurrent ovarian cancer (45). Even though the role for addition of HIPEC has not been well elucidated, the distinct biology of EOC with its propensity to spread within the peritoneal cavity and its good response to intraperitoneal chemotherapy with cisplatin and paclitaxel in optimally debulked disease, renders EOC a promising field for CRS/HIPEC (46-48).
Although large, randomized studies have shown significant efficacy of intraperitoneal chemotherapy in EOC, it has not been fully adopted in daily clinical practice, mainly because of its toxicity, complexity, and heterogeneity of delivery (48-51). While some trials exploring the role of alternative regimens for intraperitoneal chemotherapy closed early due to unacceptable rate of complications (52), some studies have shown meaningful improvement in survival with acceptable morbidity and mortality prompting an interest in repeat CRS with HIPEC (53). The benefit of HIPEC over IP catheter based chemotherapy potentially relates to more uniform distribution of the perfusate at the time of surgery, synergistic effect of heat on the action of chemotherapeutic agent, and the lack of complications noted from placement of an indwelling catheter (54).
In a single-center Italian study, 47 patients with EOC (including 25 with recurrent disease) underwent CRS and HIPEC (55). About 87.3% underwent complete CRS, with a major complication rate of 21.2% and a mortality rate of 4.2%. Mean overall survival was 30.4 months, with a mean disease free survival of 27.4 months. They noted that their morbidity/mortality rates and survival outcomes were similar to earlier series of Raspagliesi et al, Cotte et al. and Helm et al. (56-58).
Pavlov et al. published their single center experience of patients with primary as well as recurrent EOC, undergoing CRS/HIPEC (59). With a median disease-free survival of 26.2 months, median overall survival was similar for primary as well as recurrent patients (34.1 vs. 40.1 months, P>0.05). Most patients (92.8%) underwent complete CRS with a median PCI of 13.4, and morbidity and mortality rate of 17.8% and 1.8% respectively. The authors found their results comparable with a single-institution Spanish experience of 33 EOC patients, 19 primary and 14 recurrent, undergoing CRS/HIPEC for peritoneal carcinomatosis (60). Patients with complete CRS obtained survival rates of 63% at 5 years in recurrent ovarian cancer and 60% in primary ovarian cancer. Major determinants of survival were completeness of cytoreduction and negative lymph nodes.
A review by Chua et al. of 19 studies each including at least 10 or more patients, noted overall median survival from 22 to 64 months following treatment with CRS/HIPEC, disease-free survival ranging from 10 to 57 months and 5-year survival rate ranging from 12% to 66% in patients achieving optimal debulking, with acceptable morbidity and mortality (61). Similarly, a multi-institutional study from Italy that included 56 patients with a median PCI of 15.2, reported a procedure-related mortality of 5.3% and a severe morbidity rate of 26.3% (62). The median overall survival was 25.7 months while the progression-free survival was 10.8 months. ECOG status, preoperative serum albumin and completeness of cytoreduction were found to be independent prognostic factors of disease progression. Another observation was the similar overall survival of both platinum-sensitive and platinum-resistant EOC, questioning the exception of platinum-resistant recurrent disease for CRS/HIPEC.
Fagotti et al., in their single-institution prospective study, analyzed 41 patients with recurrent platinum-sensitive EOC that underwent CRS and oxaliplatin-based HIPEC (63). A disease free survival of 24 months and overall survival of 38 months was noted for the 25 patients with at least 18-month follow-up. Major complication rate was 34.8% with no mortality reported. The authors concluded that in recurrent, platinum-sensitive EOC patients, the use of CRS plus HIPEC represents a safe option, with improved survival rates compared to chemotherapy alone or surgery with standard chemotherapy. Moreover, this group demonstrated that repeat CRS with HIPEC achieved the same rate of disease free survival as compared to primary CRS, thereby demonstrating, that in optimally selected platinum-sensitive EOC patients, the chances for obtaining a meaningful disease-free interval are substantial, even after recurrent disease.
The issue of CRS/HIPEC in platinum resistant EOC patients was addressed by a larger prospective multicenter study from France (53). This study included 246 EOC patients with both platinum resistant and sensitive disease, 92.2% of whom had a complete CRS. An overall median survival of 48.9 months was noted; not significantly different for patients with platinum-resistant persistent or recurrent disease and for patients with platinum sensitive disease (48 vs. 52 months, P>0.5). Median PCI was 10.8. In patients with PCI less than 10, the completeness of CRS was the strongest prognostic indicator, while in those with PCI above 10, performance status was the only independent predictor of survival. Mortality of 0.37% and morbidity (grade III or IV) of 11.6% reported in this series, was much lower than that in other studies, likely because of the low overall PCI of patients and the extensive institutional experienced accrued by the two centers from which data was derived. Given that chemotherapy alone for the treatment of recurrent disease rarely results in median overall survival of greater than 30 months, especially in platinum-resistant disease (44,64), the authors recommend CRS/HIPEC for recurrent ovarian cancer (platinum sensitive as well as resistant), in patients with a low PCI score and in those with high PCI score and good performance status. As an update, a larger collaborative 13-institution analysis of 566 patients from the FROGHI group (French Oncologic and Gynecologic HIPEC), 474 of who had recurrent EOC, showed similar findings. Complete CRS was achieved in 74.9% of patients (65). Mortality and major morbidity rates were 0.8% and 31.3%, respectively. The median overall survival was 45.7 months for patients with recurrent EOC with no significant difference in overall survival between patients with platinum sensitive and with platinum resistant recurrence.
Despite the above studies showing benefit of CRS/HIPEC in recurrent EOC, no prospective randomized data exist to confirm the role of addition of HIPEC to CRS. Several experts have therefore advocated for prospective randomized control trials to study the role of HIPEC in primary as well as recurrent EOC (66,67). Currently, there are three ongoing randomized clinical trials (DESKTOP III, GOG 213, Dutch SOCceR trial) investigating the role of CRS with or without adjuvant chemotherapy for recurrent ovarian cancer but there are no large scale, multi-institutional randomized clinical trials investigating the role of HIPEC for recurrent EOC (68,69). In the only prospective randomized phase III study addressing this issue, investigators from Greece compared patients with advanced EOC who experienced disease recurrence after initial treatment with conservative or debulking surgery and systemic chemotherapy, by randomizing patients to two groups of 60 each based on whether they received CRS or CRS and HIPEC (70). Both groups received systemic chemotherapy after the CRS procedure. Statistically significant improved 3-year survival was shown for the CRS/HIPEC group 75% vs. 18% for the CRS only. Additionally, when comparing survival between platinum resistant and sensitive disease for the HIPEC group, no statistically significant difference was noted (26.6 vs. 26.8%, respectively); however in CRS only group, significant differences in survival between the two groups of patients was noted (15.2 vs. 10.2; P<0.002). Survival in both groups correlated with PCI and completeness of cytoreduction.
Even though level I data and RCTs are overall still lacking in the literature, there is a growing body of evidence supporting the role of CRS and HIPEC in improving survival for selected patients with recurrent EOC, irrespective of platinum sensitivity, and with acceptable morbidity and mortality (Table 5). The completeness of cytoreduction, disease burden and time to recurrence has been demonstrated to be significant factors determining overall survival.

Full table
Mesothelioma
Malignant peritoneal mesothelioma is a rare and aggressive tumor that arises from the mesothelial cells of the peritoneum and has a strong relationship with asbestos exposure (71,72). It accounts for approximately 30% of all mesotheliomas, with an estimated incidence of 400 new cases per year in the US (73,74). There are three major histopathological subtypes, each with significant difference in prognosis: epithelial, sarcomatoid and biphasic type (75-80). Classically, diffuse malignant peritoneal mesothelioma (DMPM) has been linked with a grave prognosis, with a median survival of about 12 months. Systemic chemotherapy including chemotherapeutic drugs and pemetrexed which have been used in pleural mesothelioma, have been applied to patients with DMPM with only a modest survival benefit (median overall survival 10 to 26.8 months), largely due to resistance of the cells to the cytotoxic action of these agents (81-84). In recent years however, comprehensive treatment with CRS and HIPEC has resulted in improved outcomes, with a median survival of 52-92 months and 5-year survival rate of 39-63%, as compared with 9-12 months in historic series treated by palliative surgery and systemic chemotherapy (75,85-89). Due to the rarity of the disease, data regarding prognosis have been derived mainly from single- and multi-institutional series with no randomized trials to elucidate the optimum treatment strategy. As a result, there are no established standards of care for patients with this condition. Ongoing research is further attempting to identify molecular features that can correlate with improved response to treatment (90,91), however until improved treatment strategies are proven to be beneficial, treatment for patients with DMPM has been based largely on consensus guidelines (92). Despite relatively encouraging results of aggressive loco-regional treatment with maximal cytoreduction, disease recurrence occurs in more than half the patients (75,85-89). In a majority of these patients, treatment failure is largely isolated to the peritoneal cavity (93). This has created an interest in repeat CRS/HIPEC for selected patients who develop recurrence.
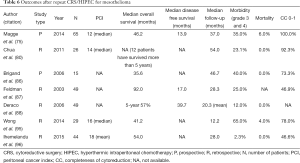
In one of the earliest studies, Golse et al. reported outcomes of 30 patients undergoing repeat CRS/HIPEC for isolated peritoneal recurrences, 3 of who had mesothelioma (11). Median interval between initial and second-time CRS with HIPEC was 22 months; most patients underwent a complete CRS with a major morbidity rate of 40% and a mortality rate of 3.3%.
In their study of 79 patients undergoing iterative CRS, Chua et al. included 9 patients with a diagnosis of DMPM (94). Although perioperative outcomes were not individually analyzed for mesothelioma patients, no mortality and a 41% major morbidity rate was observed in the iterative CRS/HIPEC group. The median, 3- and 5-year survival was 57 months, 80% and 27% respectively. Lower age, interval from primary to repeat CRS/HIPEC of greater than 18 months, administration of HIPEC and lesser number of bowel resections were identified as independent predictors of improved overall survival.
In a recent small report by Wong et al., 8 patients underwent repeat CRS/HIPEC, with one of them undergoing a third HIPEC and another undergoing additional three iterative procedures (95). All of the patients that underwent a repeat HIPEC had a complete CRS and a PCI score less than 20. Median overall survival was higher for the repeat HIPEC group at 80 vs. 27.2 months for the single HIPEC group while the postoperative complication rate was comparable with 50% for the repeat HIPEC group vs. 65% in the single HIPEC group. PCI <20 and low completeness of cytoreduction scores were independently associated with improved outcomes.
In another study published by the Ihemelandu et al., 44 (21.5%) of 205 patients that initially underwent CRS/HIPEC, had a repeat procedure for DMPM (96). The median overall survival of patients undergoing an iterative CRS and HIPEC was 54 months versus 77 months following an initial CRS and HIPEC (P=0.96), with a low rate (2.3%) of major morbidity and no perioperative mortalities. Histopathological type, HIPEC regimen employed, near complete cytoreduction, female sex, age and absence of postoperative complications were identified as independent predictors of overall survival on multivariate analysis. The authors concluded that repeat CRS/HIPEC can be offered to select patients and offers survival benefit if near complete cytoreduction is obtained.
Huang et al recently reported their outcomes of patients with DMPM undergoing CRS/HIPEC. Ten of 44 patients that underwent an initial procedure underwent a second CRS/HIPEC (3). The median survival for those who only had one CRS/HIPEC was 22.0 months in comparison to 62 months for patients undergoing iterative procedures, with a median time of 15.2 months between the first and second operations. Major complication rate was 60% with no mortalities reported for iterative CRS/HIPEC. The authors recommended repeat CRS/HIPEC in selected patients with recurrent DMPM and referral to specialized centers with significant experience in treating such patients.
In our study reviewing the data on repeat CRS/HIPEC, there were 7 patients with recurrent DMPM and the median overall survival for this subgroup was 52.9 months after both procedures, with an observed additional median survival benefit of 21.8 (range, 3.1-44.1) months from the second HIPEC (10). A 48% complication rate was observed in patients undergoing repeat HIPEC, similar to the 35% initial complication rate.
Despite the benefit of repeat CRS/HIPEC for DMPM, robust data to establish suitable criteria for selection of patients likely to benefit from an iterative procedure are lacking. Given the rarity of the disease, carefully designed multicenter trials are necessary to elucidate this issue. However, repeat CRS/HIPEC does hold promise for select patients with recurrent disease (Table 6).
Full table
Conclusions
Despite the variability of the above studies, they all demonstrate that repeat CRS/HIPEC is a feasible and safe procedure with similar morbidity and mortality as the initial cytoreduction. Repeat CRS/HIPEC offers encouraging survival results in patients with low volume, isolated peritoneal recurrence and at least a year-long disease free interval from the initial CRS/HIPEC. Therefore selection criteria for surgical candidates should be predominantly based on favorable tumor biology and ability to achieve a complete macroscopic CRS. Further multi-institutional trials are needed to establish standardized criteria for the management of patients with recurrent peritoneal carcinomatosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Levine EA, Stewart JH 4th, Shen P, et al. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg 2014;218:573-85. [PubMed]
- Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008;15:2426-32. [PubMed]
- Huang Y, Alzahrani NA, Liauw W, et al. Repeat cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for recurrent diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol 2015;41:1373-8. [PubMed]
- Verwaal VJ, Boot H, Aleman BM, et al. Recurrences after peritoneal carcinomatosis of colorectal origin treated by cytoreduction and hyperthermic intraperitoneal chemotherapy: location, treatment, and outcome. Ann Surg Oncol 2004;11:375-9. [PubMed]
- Chua TC, Liauw W, Morris DL. Early recurrence of pseudomyxoma peritonei following treatment failure of cytoreductive surgery and perioperative intraperitoneal chemotherapy is indicative of a poor survival outcome. Int J Colorectal Dis 2012;27:381-9. [PubMed]
- Bijelic L, Yan TD, Sugarbaker PH. Treatment failure following complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from colorectal or appendiceal mucinous neoplasms. J Surg Oncol 2008;98:295-9. [PubMed]
- Königsrainer I, Horvath P, Struller F, et al. Risk factors for recurrence following complete cytoreductive surgery and HIPEC in colorectal cancer-derived peritoneal surface malignancies. Langenbecks Arch Surg 2013;398:745-9. [PubMed]
- Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012;30:2449-56. [PubMed]
- Yan TD, Black D, Savady R, et al. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol 2007;14:484-92. [PubMed]
- Votanopoulos KI, Ihemelandu C, Shen P, et al. Outcomes of repeat cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for the treatment of peritoneal surface malignancy. J Am Coll Surg 2012;215:412-7. [PubMed]
- Golse N, Bakrin N, Passot G, et al. Iterative procedures combining cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal recurrence: postoperative and long-term results. J Surg Oncol 2012;106:197-203. [PubMed]
- Wong JF, Tan GH, Wang W, et al. Repeat Cytoreductive Surgery and HIPEC for peritoneal surface malignancy and peritoneal carcinomatosis. World J Surg 2015;39:1578-83. [PubMed]
- Bradley RF, Stewart JH 4th, Russell GB, et al. Pseudomyxoma peritonei of appendiceal origin: a clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol 2006;30:551-9. [PubMed]
- Bradley RF, Cortina G, Geisinger KR. Pseudomyxoma peritonei: review of the controversy. Curr Diagnostic Pathol 2007;13:410-6.
- Ronnett BM, Zahn CM, Kurman RJ, et al. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol 1995;19:1390-408. [PubMed]
- Gough DB, Donohue JH, Schutt AJ, et al. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg 1994;219:112-9. [PubMed]
- Esquivel J, Sugarbaker PH. Second-look surgery in patients with peritoneal dissemination from appendiceal malignancy: analysis of prognostic factors in 98 patients. Ann Surg 2001;234:198-205. [PubMed]
- Yan TD, Bijelic L, Sugarbaker PH. Critical analysis of treatment failure after complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from appendiceal mucinous neoplasms. Ann Surg Oncol 2007;14:2289-99. [PubMed]
- Mohamed F, Chang D, Sugarbaker PH. Third look surgery and beyond for appendiceal malignancy with peritoneal dissemination. J Surg Oncol 2003;83:5-12; discussion 12-3. [PubMed]
- Miner TJ, Shia J, Jaques DP, et al. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg 2005;241:300-8. [PubMed]
- Sardi A, Jimenez WA, Nieroda C, et al. Repeated cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from appendiceal cancer: analysis of survival outcomes. Eur J Surg Oncol 2013;39:1207-13. [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [PubMed]
- Chu DZ, Lang NP, Thompson C, et al. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer 1989;63:364-7. [PubMed]
- Jayne DG, Fook S, Loi C, et al. Peritoneal carcinomatosis from colorectal cancer. Br J Surg 2002;89:1545-50. [PubMed]
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737-43. [PubMed]
- Cao C, Yan TD, Black D, et al. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2009;16:2152-65. [PubMed]
- Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 2009;27:681-5. [PubMed]
- Franko J, Ibrahim Z, Gusani NJ, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer 2010;116:3756-62. [PubMed]
- Esquivel J, Sticca R, Sugarbaker P, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann Surg Oncol 2007;14:128-33. [PubMed]
- Cashin PH, Graf W, Nygren P, et al. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis: prognosis and treatment of recurrences in a cohort study. Eur J Surg Oncol 2012;38:509-15. [PubMed]
- Portilla AG, Sugarbaker PH, Chang D. Second-look surgery after cytoreduction and intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer: analysis of prognostic features. World J Surg 1999;23:23-9. [PubMed]
- Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284-92. [PubMed]
- Kianmanesh R, Scaringi S, Sabate JM, et al. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Ann Surg 2007;245:597-603. [PubMed]
- Bijelic L, Yan TD, Sugarbaker PH. Failure analysis of recurrent disease following complete cytoreduction and perioperative intraperitoneal chemotherapy in patients with peritoneal carcinomatosis from colorectal cancer. Ann Surg Oncol 2007;14:2281-8. [PubMed]
- Goéré D, Souadka A, Faron M, et al. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol 2015;22:2958-64. [PubMed]
- Williams BH, Alzahrani NA, Chan DL, et al. Repeat cytoreductive surgery (CRS) for recurrent colorectal peritoneal metastases: yes or no? Eur J Surg Oncol 2014;40:943-9. [PubMed]
- Braam HJ, van Oudheusden TR, de Hingh IH, et al. Patterns of recurrence following complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. J Surg Oncol 2014;109:841-7. [PubMed]
- Hogg R, Friedlander M. Biology of epithelial ovarian cancer: implications for screening women at high genetic risk. J Clin Oncol 2004;22:1315-27. [PubMed]
- American Cancer Society. Cancer facts & figures 2015. Atlanta: American Cancer Society; 2015.
- Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 2002;20:1248-59. [PubMed]
- Rubin SC, Hoskins WJ, Hakes TB, et al. Recurrence after negative second-look laparotomy for ovarian cancer: analysis of risk factors. Am J Obstet Gynecol 1988;159:1094-8. [PubMed]
- Cannistra SA. Cancer of the ovary. N Engl J Med 2004;351:2519-29. [PubMed]
- Galaal K, Naik R, Bristow RE, et al. Cytoreductive surgery plus chemotherapy versus chemotherapy alone for recurrent epithelial ovarian cancer. Cochrane Database Syst Rev 2010.CD007822. [PubMed]
- Bristow RE, Puri I, Chi DS. Cytoreductive surgery for recurrent ovarian cancer: a meta-analysis. Gynecol Oncol 2009;112:265-74. [PubMed]
- Al Rawahi T, Lopes AD, Bristow RE, et al. Surgical cytoreduction for recurrent epithelial ovarian cancer. Cochrane Database Syst Rev 2013;2:CD008765. [PubMed]
- Helm CW, Bristow RE, Kusamura S, et al. Hyperthermic intraperitoneal chemotherapy with and without cytoreductive surgery for epithelial ovarian cancer. J Surg Oncol 2008;98:283-90. [PubMed]
- Chi DS, Eisenhauer EL, Zivanovic O, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol 2009;114:26-31. [PubMed]
- Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006;354:34-43. [PubMed]
- Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 1996;335:1950-5. [PubMed]
- Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the gynecol. J Clin Oncol 2001;19:1001-7. [PubMed]
- Smith HO, Moon J, Wilczynski SP, et al. Southwest Oncology Group Trial S9912: intraperitoneal cisplatin and paclitaxel plus intravenous paclitaxel and pegylated liposomal doxorubicin as primary chemotherapy of small-volume residual stage III ovarian cancer. Gynecol Oncol 2009;114:206-9. [PubMed]
- Pomel C, Ferron G, Lorimier G, et al. Hyperthermic intra-peritoneal chemotherapy using oxaliplatin as consolidation therapy for advanced epithelial ovarian carcinoma. Results of a phase II prospective multicentre trial. CHIPOVAC study. Eur J Surg Oncol 2010;36:589-93. [PubMed]
- Bakrin N, Cotte E, Golfier F, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for persistent and recurrent advanced ovarian carcinoma: a multicenter, prospective study of 246 patients. Ann Surg Oncol 2012;19:4052-8. [PubMed]
- Sugarbaker PH. Laboratory and clinical basis for hyperthermia as a component of intracavitary chemotherapy. Int J Hyperthermia 2007;23:431-42. [PubMed]
- Di Giorgio A, Naticchioni E, Biacchi D, et al. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer 2008;113:315-25. [PubMed]
- Raspagliesi F, Kusamura S, Campos Torres JC, et al. Cytoreduction combined with intraperitoneal hyperthermic perfusion chemotherapy in advanced/recurrent ovarian cancer patients: The experience of National Cancer Institute of Milan. Eur J Surg Oncol 2006;32:671-5. [PubMed]
- Cotte E, Glehen O, Mohamed F, et al. Cytoreductive surgery and intraperitoneal chemo-hyperthermia for chemo-resistant and recurrent advanced epithelial ovarian cancer: prospective study of 81 patients. World J Surg 2007;31:1813-20. [PubMed]
- Helm CW, Randall-Whitis L, Martin RS 3rd, et al. Hyperthermic intraperitoneal chemotherapy in conjunction with surgery for the treatment of recurrent ovarian carcinoma. Gynecol Oncol 2007;105:90-6. [PubMed]
- Pavlov MJ, Kovacevic PA, Ceranic MS, et al. Cytoreductive surgery and modified heated intraoperative intraperitoneal chemotherapy (HIPEC) for advanced and recurrent ovarian cancer -- 12-year single center experience. Eur J Surg Oncol 2009;35:1186-91. [PubMed]
- Rufián S, Muñoz-Casares FC, Briceño J, et al. Radical surgery-peritonectomy and intraoperative intraperitoneal chemotherapy for the treatment of peritoneal carcinomatosis in recurrent or primary ovarian cancer. J Surg Oncol 2006;94:316-24. [PubMed]
- Chua TC, Robertson G, Liauw W, et al. Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery in ovarian cancer peritoneal carcinomatosis: systematic review of current results. J Cancer Res Clin Oncol 2009;135:1637-45. [PubMed]
- Deraco M, Virzì S, Iusco DR, et al. Secondary cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for recurrent epithelial ovarian cancer: a multi-institutional study. BJOG 2012;119:800-9. [PubMed]
- Fagotti A, Costantini B, Vizzielli G, et al. HIPEC in recurrent ovarian cancer patients: morbidity-related treatment and long-term analysis of clinical outcome. Gynecol Oncol 2011;122:221-5. [PubMed]
- Mutch DG. Gemcitabine combination chemotherapy of ovarian cancer. Gynecol Oncol 2003;90:S16-20. [PubMed]
- Bakrin N, Bereder JM, Decullier E, et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol 2013;39:1435-43. [PubMed]
- Chua TC, Liauw W, Robertson G, et al. Towards randomized trials of cytoreductive surgery using peritonectomy and hyperthermic intraperitoneal chemotherapy for ovarian cancer peritoneal carcinomatosis. Gynecol Oncol 2009;114:137-9. [PubMed]
- Markman M. Hyperthermic intraperitoneal chemotherapy in the management of ovarian cancer: A critical need for an evidence-based evaluation. Gynecol Oncol 2009;113:4-5. [PubMed]
- van de Laar R, Zusterzeel PL, Van Gorp T, et al. Cytoreductive surgery followed by chemotherapy versus chemotherapy alone for recurrent platinum-sensitive epithelial ovarian cancer (SOCceR trial): a multicenter randomised controlled study. BMC Cancer 2014;14:22. [PubMed]
- van de Laar R, Massuger LF, Van Gorp T, et al. External validation of two prediction models of complete secondary cytoreductive surgery in patients with recurrent epithelial ovarian cancer. Gynecol Oncol 2015;137:210-5. [PubMed]
- Spiliotis J, Halkia E, Lianos E, et al. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol 2015;22:1570-5. [PubMed]
- Boffetta P. Epidemiology of peritoneal mesothelioma: a review. Ann Oncol 2007;18:985-90. [PubMed]
- Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005;353:1591-603. [PubMed]
- Tanida S, Kataoka H, Kubota E, et al. Combination chemotherapy with cisplatin and gemcitabine in malignant peritoneal mesothelioma. Int J Clin Oncol 2009;14:266-9. [PubMed]
- Yan TD, Brun EA, Cerruto CA, et al. Prognostic indicators for patients undergoing cytoreductive surgery and perioperative intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma. Ann Surg Oncol 2007;14:41-9. [PubMed]
- Magge D, Zenati MS, Austin F, et al. Malignant peritoneal mesothelioma: prognostic factors and oncologic outcome analysis. Ann Surg Oncol 2014;21:1159-65. [PubMed]
- Munkholm-Larsen S, Cao CQ, Yan TD. Malignant peritoneal mesothelioma. World J Gastrointest Surg 2009;1:38-48. [PubMed]
- Schaub NP, Alimchandani M, Quezado M, et al. A novel nomogram for peritoneal mesothelioma predicts survival. Ann Surg Oncol 2013;20:555-61. [PubMed]
- Mirarabshahii P, Pillai K, Chua TC, et al. Diffuse malignant peritoneal mesothelioma--an update on treatment. Cancer Treat Rev 2012;38:605-12. [PubMed]
- Yan TD, Deraco M, Elias D, et al. A novel tumor-node-metastasis (TNM) staging system of diffuse malignant peritoneal mesothelioma using outcome analysis of a multi-institutional database*. Cancer 2011;117:1855-63. [PubMed]
- Chua TC, Yan TD, Deraco M, et al. Multi-institutional experience of diffuse intra-abdominal multicystic peritoneal mesothelioma. Br J Surg 2011;98:60-4. [PubMed]
- Jänne PA, Wozniak AJ, Belani CP, et al. Open-label study of pemetrexed alone or in combination with cisplatin for the treatment of patients with peritoneal mesothelioma: outcomes of an expanded access program. Clin Lung Cancer 2005;7:40-6. [PubMed]
- Carteni G, Manegold C, Garcia GM, et al. Malignant peritoneal mesothelioma-Results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent. Lung Cancer 2009;64:211-8. [PubMed]
- Simon GR, Verschraegen CF, Jänne PA, et al. Pemetrexed plus gemcitabine as first-line chemotherapy for patients with peritoneal mesothelioma: final report of a phase II trial. J Clin Oncol 2008;26:3567-72. [PubMed]
- Le DT, Deavers M, Hunt K, et al. Cisplatin and irinotecan (CPT-11) for peritoneal mesothelioma. Cancer Invest 2003;21:682-9. [PubMed]
- Sugarbaker PH, Welch LS, Mohamed F, et al. A review of peritoneal mesothelioma at the Washington Cancer Institute. Surg Oncol Clin N Am 2003;12:605-21. xi. [PubMed]
- Brigand C, Monneuse O, Mohamed F, et al. Peritoneal mesothelioma treated by cytoreductive surgery and intraperitoneal hyperthermic chemotherapy: results of a prospective study. Ann Surg Oncol 2006;13:405-12. [PubMed]
- Feldman AL, Libutti SK, Pingpank JF, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol 2003;21:4560-7. [PubMed]
- Deraco M, Nonaka D, Baratti D, et al. Prognostic analysis of clinicopathologic factors in 49 patients with diffuse malignant peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol 2006;13:229-37. [PubMed]
- Yan TD, Welch L, Black D, et al. A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignancy peritoneal mesothelioma. Ann Oncol 2007;18:827-34. [PubMed]
- Baratti D, Kusamura S, Deraco M. Diffuse malignant peritoneal mesothelioma: systematic review of clinical management and biological research. J Surg Oncol 2011;103:822-31. [PubMed]
- Foster JM, Gatalica Z, Lilleberg S, et al. Novel and existing mutations in the tyrosine kinase domain of the epidermal growth factor receptor are predictors of optimal resectability in malignant peritoneal mesothelioma. Ann Surg Oncol 2009;16:152-8. [PubMed]
- Deraco M, Bartlett D, Kusamura S, et al. Consensus statement on peritoneal mesothelioma. J Surg Oncol 2008;98:268-72. [PubMed]
- Baratti D, Kusamura S, Cabras AD, et al. Diffuse malignant peritoneal mesothelioma: Failure analysis following cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol 2009;16:463-72. [PubMed]
- Chua TC, Quinn LE, Zhao J, et al. Iterative cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for recurrent peritoneal metastases. J Surg Oncol 2013;108:81-8. [PubMed]
- Wong J, Koch AL, Deneve JL, et al. Repeat cytoreductive surgery and heated intraperitoneal chemotherapy may offer survival benefit for intraperitoneal mesothelioma: a single institution experience. Ann Surg Oncol 2014;21:1480-6. [PubMed]
- Ihemelandu C, Bijelic L, Sugarbaker PH. Iterative cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for recurrent or progressive diffuse malignant peritoneal mesothelioma: clinicopathologic characteristics and survival outcome. Ann Surg Oncol 2015;22:1680-5. [PubMed]


