Palliative cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion: current clinical practice or misnomer?
Peritoneal carcinomatosis remains one of the greatest challenges in cancer treatment. Regardless of the origin of the cancer, the diagnosis of peritoneal carcinomatosis is almost uniformly associated with a terminal and limited prognosis. In cancers for which there are limited effective treatment options (e.g., gastric, pancreas, cholangiocarcinoma), this expectation is not unrealistic (1). Even in cancers for which there are effective systemic therapies (e.g., colon, breast), patients who develop peritoneal carcinomatosis tend to fare less well than patients with non-peritoneal metastases (2,3). Consequently, treatments offered are frequently limited to “palliative systemic therapy”-chemotherapy given for some survival benefit, but not curative intent (and hopefully with an early referral to palliative medicine for supportive management and assistance with goals of care) (4,5).
However, not all peritoneal carcinomatoses are created equal. Recent advances in our understanding of carcinomatosis demonstrate that it is not a single entity with a uniformly lethal behavior (6). This is particularly true for metastatic cancers that are often isolated to the peritoneal cavity (e.g., low grade appendiceal cancer, platin-sensitive ovarian cancer, well-differentiated epithelioid peritoneal mesothelioma, colon cancer). Furthermore, due to significant advances in surgical techniques and regional therapies, “palliative systemic therapy” is no longer the only treatment option available.
Since the 1980s surgical oncologists have been developing and refining a combined treatment approach of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion (CRS/HIPEC) for patients with isolated peritoneal carcinomatosis (7). The rationale behind this approach is to achieve a macroscopically complete cytoreduction of the carcinomatosis (no residual tumor greater than 0.25 cm thick) then treat any residual disease with high concentration, hyperthermic chemotherapy applied directly into the peritoneal cavity. Achieving a complete cytoreduction often involves major abdominal surgery. Based upon the volume and distribution of the carcinomatosis, as well as the “invasiveness” of the peritoneal implants, achieving a complete cytoreduction may involve peritoneal stripping, multi-visceral resections (often including the omentum, large and small bowel, stomach, spleen, gallbladder, uterus and ovaries, pancreas, ureters, bladder) and the creation of temporary or permanent stomas.
Upon completion of the cytoreduction, HIPEC is typically administered for 30-120 minutes (depending upon the preference of the surgical oncologist and type of chemotherapy used). Hyperthermia is added to the treatment for three reasons. First, cancer cells are more sensitive to heat (typically at >43 °C for 1 hour) than normal cells (8-10). Second, a synergistic increase in cytotoxicity occurs between hyperthermia and many chemotherapy agents (11,12). Third, it is theorized that hyperthermia facilitates deeper penetration of the chemotherapy into the peritoneal tissues. The whole treatment usually takes an average of 8-10 hours. Most patients are admitted to an intensive care unit immediately after surgery and the average length of stay in the hospital ranges from 8-22 days (13-16).
Surgery of this magnitude, combined with heated chemotherapy, cannot be performed without risk of complications, including a risk of dying. Over time and with experience, the admittedly high rate of surgical complications and deaths that accompanied the early use of CRS/HIPEC has improved dramatically. A comprehensive review of reported results from over 20 CRS/HIPEC centers since 2003 shows current major morbidity rates of 0-50% and mortality rates between 0-6% at high volume centers (17). These rates are now consistent with other major abdominal cancer operations such as pancreaticoduodenectomy and esophagogastrectomy (18).
Despite a lack of level I evidence and endorsement by the medical oncology community, the use of CRS/HIPEC has continued to expand (19,20). Today it is largely considered the standard of care treatment for low grade mucinous tumors of the appendix (21). It is also gaining acceptance as part of the multimodality treatment of selected patients with isolated carcinomatosis from colorectal cancer and peritoneal mesothelioma (22,23). Recent reports of long term outcomes in appendiceal cancer demonstrate a significant improvement in 5- and 10-year survival rates (81% and 70%) in patients with low grade mucinous tumors of the appendix as compared with CRS alone (17,24). For patients with peritoneal mesotheliomas who undergo complete cytoreduction and HIPEC, mean survivals of 38 to over 90 months are reported compared with 12 months for those who receive systemic therapy alone (25,26). For patients with isolated, limited, peritoneal carcinomatosis from colon cancer who undergo a complete cytoreduction and HIPEC, reported median survivals of 32-63 months and 44-58% 5-year survival compare favorably with an expected median survival of 5.2-23.9 months and 5-year survival of 0-19% with systemic therapy alone (27-32) (Table 1). The American Society of Peritoneal Surface Malignancies (ASPSM) has published an opinion statement establishing an expectation of a median survival of 30 months after CRS/HIPEC for patients with colorectal cancer (33).

Full table
Unfortunately, as encouraging as these results appear for a condition that is usually considered lethal, not all patients achieve these survival benefits. Even for patients in whom a complete cytoreduction is achieved, the risk of cancer recurrence can be quite high depending upon the primary cancer. Oftentimes the expectation from the time of surgery is that the CRS/HIPEC will be therapeutic (e.g., offer some survival benefit), but not be curative. Thus by analogy with the medical oncology nomenclature of “palliative systemic therapy” given for metastatic or recurrent disease with the intent of some survival benefit but not cure (regardless of the presence or absence of symptoms), many (if not most) CRS/HIPEC procedures could be considered “palliative”. However, for a number of reasons, the term “palliative CRS/HIPEC” is not currently used in either the literature or clinically in the context of a CRS/HIPEC with complete cytoreduction.
First, the term “palliative” continues to strike fear in the hearts of patients, families and providers. It is often taken as a sign that there is “no hope” and can negate even an expected significant survival benefit if the goal of treatment is not curative. It is the authors’ opinion and experience that even if the surgical oncologist tells the patient and family that the intent of the CRS/HIPEC is not curative, they still hope for something better. Although many providers call this “denial” and are uncomfortable with what they perceive to be unrealistic expectations, this hope is a natural part of processing an overwhelming circumstance, and may actually play a role in the quality of life (QOL) after the surgery (29,31,32,34,35). Calling the CRS/HIPEC palliative may diminish this hope for some patients.
Second, from a surgical standpoint, the concept of “palliative surgery” is conventionally reserved for patients with symptoms that can potentially be relieved with a surgical intervention. For patients with symptomatic cancers, the intent of the surgical intervention is not focused on treating the cancer, but relieving the symptoms. On occasion, this may result in an improvement in survival, although that is not the intended goal per se. Current recommendations are that major palliative surgical interventions only be offered to patients with an adequate life expectancy and with minimal risks (36). Although a typical CRS/HIPEC performed with the intent of survival benefit would rarely qualify as a minimal risk procedure, there does appear to be a role for palliative HIPEC in the management of patients with malignant ascites (30,37-43). In addition, as high volume HIPEC centers become more experienced with CRS/HIPEC, the potential role of “palliative CRS/HIPEC” in the management of peritoneal carcinomatosis is being raised. Specifically, some are beginning to question whether there is a role for CRS/HIPEC in the setting of near-complete cytoreduction, ostensibly to prevent or delay future symptomatic cancer-related complications—an approach starting to be referred to as “palliative CRS/HIPEC”.
Either improved survival or improved QOL is an essential requirement for the rational administration of cancer-directed treatment. The ideal treatment would accomplish both, or at least improve survival without decreasing any aspect of a person’s QOL. Because it is both an easily measurable and highly desirable endpoint, survival is considered the gold standard to determine the efficacy of cancer treatment. Many treatment recommendations by providers and decisions to pursue treatment by patients are made on the basis of survival benefit alone-regardless of the impact on QOL. This includes recommendations and decisions around CRS/HIPEC. However, all patients who undergo CRS/HIPEC (curative or “palliative”) incur some impact on their QOL. Given the limited prognosis for most patients with carcinomatosis, one of the most important questions regarding the use of CRS/HIPEC is whether or not the potential benefits, including improved survival or QOL, outweigh the risks of either a surgical complication, permanently diminished QOL or even death.
Although harder to measure than survival, understanding the impact of cancer-directed therapy on QOL is extremely valuable to help providers and patients make well-informed decisions about pursuing treatment as well as to help manage and limit the adverse effects. For patients suffering side effects of an advanced cancer, chemotherapy, radiation, surgery or other procedures given for truly palliative intent can significantly improve QOL. Furthermore, although such interventions may not be intended to improve survival, recent studies have shown that initiation of early, concurrent, palliative care not only improves QOL but may also improve survival-even in the setting of less cancer-directed therapy (44). On the other hand, cancer-directed therapy being administered with the goal of improving survival often diminishes some aspect of a patient’s QOL. This impact is usually considered an acceptable consequence for the goal of improved survival. Given the magnitude and complexity of CRS/HIPEC, its associated morbidity and mortality, and the limited potential survival benefit for many cancers, this is one of the most extreme settings in which weighing the value of potential survival benefit against the potential loss of QOL is played out on a daily basis.
Although still in its infancy, there is a growing body of literature regarding QOL after CRS/HIPEC. Since 2001, there have been at least 22 original publications in which validated QOL instruments were used to investigate the impact of CRS/HIPEC on QOL (Table 2) (29,31,32,34,35,45-61). The study sizes range from 10-216 patients (average 60), with 10 having less than 50 patients. Most studies (n=17) include a wide variety of primary cancers. CRS/HIPEC techniques, HIPEC chemotherapy agents, and morbidity and mortality rates also varied widely. Timing of the surveys and questionnaires are different between the studies. Only 15 studies included a pre-operative assessment to establish a baseline QOL. Five reported QOL that was back to baseline for more than half of the patients by 3 months (31,46,52,54,60). Four reported QOL that was higher than baseline at 6 months, with only 2 reporting QOL below baseline at 6 months (31,48,54,56,60). Of the 10 studies reporting QOL at 12 months, 5 reported increased QOL relative to baseline and the remaining 5 were at baseline (Table 3). Based upon these findings, most authors have concluded that CRS/HIPEC has an acceptable transient negative impact on overall QOL with many patients experiencing an improved QOL (if they survive long enough). However, there are a number of limitations of these data that need to be considered in order to get a fuller picture of the impact of CRS/HIPEC on QOL.
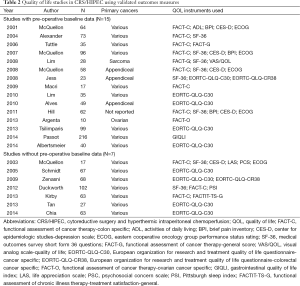
Full table
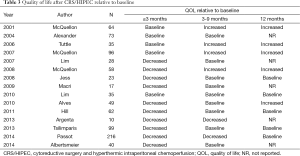
Full table
An inherent challenge in all QOL research is the rate of survey completion over time. It is readily recognized that as patients’ clinical conditions worsen, they are less likely to complete QOL surveys. Also, with a disease with such a poor prognosis as peritoneal carcinomatosis, there is an expected rate of attrition due to deaths. Both of these factors have likely played a role in the QOL after CRS/HIPEC studies. In one study by McQuellon et al., there was a 32% mortality rate at 12 months (54).
Of the 15 studies with baseline QOL data, 9 reported the survey completion rate at each time point (29,31,47,49,52,54,56,59,60). In four studies, the baseline data was not complete. The average rate of completion was 72% (40-100%) at 3 months and 51% (25-92%) at 12 months. Given the small size of most of the study populations (average =54, range =13-96), such attrition resulted in a relatively low actual number of surveys completed per study at the later time points (range, 11-45) making meaningful statistical analysis difficult. Furthermore, it is assumed that the patients who filled out the surveys at the later dates were both alive and well enough to do so. Thus, even with the use of analytic techniques that account for data that are missing, the data most likely reflect an overestimation of the true overall QOL.
It is also important to consider the relevance of clinical and statistical significance in this patient population. Given the small sample sizes, a difference may not reach statistical significance but may be perceived by the patient as beneficial or detrimental (62). Thus rather than looking for statistically significant differences in the survey results, investigators and clinicians who use QOL assessments in practice may gain more valuable information from identifying the minimally important difference (MID)-the smallest change in a patient related outcome measure that is perceived by patients as beneficial or that will result in a change in treatment (63). Of note, and as pointed out by McQuellon et al., changes that are statistically significant at one year are likely to be clinically significant as well (62).
Another factor that must be considered is that the baseline scores for patients with peritoneal carcinomatosis tend to be low relative to a normal population given the diagnosis, prognosis and symptoms. McQuellon has shown that patients with symptoms at baseline were more likely to report improved QOL early, while those without symptoms often expressed a worse QOL after CRS/HIPEC (31). This may apply to existential or emotional pain as well. In a study by Tan et al., the authors found that cognitive function and fatigue were better than a reference group of cancer-free patients at 6 months after HIPEC. They hypothesized that this might be due to post traumatic growth (the gradual internal paradigm shifts that may occur in a person following internal disruption precipitated by traumatic events) (32). A second study by the same group also reported improved QOL scores in patients with recurrence after HIPEC as compared to both a cancer-free survival group and the EORTC control group (34). The authors attributed these findings to the hope afforded patients by CRS/HIPEC. One potential confounding factor of these two studies was the use of an interviewer to facilitate completion of the survey. It should also be recognized that these studies were specifically looking at QOL outcomes in an Asian population.
While the overall picture seems to be one of adequate recovery, some patients report feeling the effects of CRS/HIPEC for a year or longer. While most studies report a return to baseline of symptoms such as nausea, vomiting, pain, anorexia and weight loss by 3 months, some report a persistent decrease in physical and functional domains lasting up to a year (31,51,54,59). Some of the most common reasons for persistent patient-reported decreased QOL after CRS/HIPEC included gastrointestinal symptoms, fatigue, sleep disturbances and depression (35,51,54,56,58,59). The gastrointestinal symptoms typically include bloating, diarrhea and constipation. Reports of depression at some time within the first year after CRS/HIPEC were as high as 50% in one study (29). Because of the high prevalence and persistence of depression after CRS/HIPEC, McQuellon and colleagues at the Wake Forest Medical Center (the HIPEC center with the most published CRS/HIPEC QOL experience) have adopted a program of referring patients for a psychological evaluation if they have a CES-D score ≥17 (54). The presence of a stoma also had a significant impact on QOL at later time points in a study by Schmidt et al., but was not found to be significant in three other studies (29,57,58,61). Despite these often profound and life-changing outcomes, a study by Kirby et al. found that 79% of patients said that they would undergo CRS/HIPEC again (50).
Awareness of the expected time course of the resolution of the acute side effects and potential for lingering side effects after CRS/HIPEC is essential for providers to optimize the comprehensive recovery of their patients. It is also important to guide efforts to decrease the impact of CRS/HIPEC on QOL. Length of surgery, volume of tumor burden and the requirement for major resections has been suggested as possible factors related to the decreased QOL after CRS/HIPEC (57,58). Interestingly, these factors have also been associated with poorer survival outcomes (64). Combined with the low morbidity and mortality in the reports on HIPEC for palliation of ascites, the data would suggest that the greatest impact on QOL after CRS/HIPEC comes from the CRS itself.
The current literature suggests that HIPEC in the setting of an incomplete cytoreduction does not offer any advantage in terms of overall survival (14,64,65). Because of this, Elias and Goere have gone so far as to say that it is “unethical, dangerous, costly and finally reprehensible” to perform HIPEC in patients with an incomplete cytoreduction due to the added morbidity of the procedure (66). As mentioned earlier, with increasing experience at high volume HIPEC centers, there is a growing interest amongst HIPEC surgeons regarding a potential role for HIPEC in the setting of a near-complete cytoreduction—an approach increasingly referred to as palliative CRS/HIPEC. To date, there are no survival or QOL data to support this approach.
One setting in which HIPEC does appear to offer benefit even if a complete cytoreduction cannot be achieved is for patients with malignant ascites. A number of studies have demonstrated the efficacy of HIPEC in the treatment of malignant ascites (30,37-39,41-43) (Table 4). In all of these studies, the main outcome measure was the resolution of ascites. There were no deaths related to the surgery and only minimal morbidity reported. Due to the advanced nature of the disease, the median survivals were relatively short (3-9 months). All of the studies reported either persistent resolution of the ascites for the duration of the patients’ survival or at the time of last follow-up. None of these studies included validated QOL surveys as part of their assessment. While it is hard to know the true impact of the HIPEC on overall QOL, many of these studies reported that patients no longer required repeat paracentesis as well as significant improvement in Karnofsky performance scores. As there is often no CRS involved, laparoscopic and even ultrasound-guided approached to palliative HIPEC have evolved. Even though these are relatively less invasive, given the need for general anesthesia and a hospital stay, it is reasonable to reserve this approach for patients with a longer life expectancy and higher performance status according to the recommendations for palliative surgical interventions. It will also become increasingly important to consider the relative cost-effectiveness of these procedures in the larger clinical context.

Full table
Is “palliative CRS/HIPEC” already part of current clinical practice or is it a misnomer? The answer is yes, depending upon the intention of the word palliative. Palliative HIPEC (with or without CRS) can be used to treat malignant ascites thereby palliating the symptoms of abdominal discomfort and distention, dyspnea and sometimes early satiety. It can also help patients avoid repeated paracentesis or the need for an intraperitoneal drainage catheter. The term “palliative CRS/HIPEC”, if used in the same context as palliative systemic chemotherapy given to patients with metastatic cancer for therapeutic (survival benefit) but not curative intent, already describes many, if not most, CRS/HIPECs performed. Although there are growing reports of long-term survivors after CRS/HIPEC with a complete cytoreduction for challenging cancers like colorectal cancer and peritoneal mesothelioma, for most patients the likelihood of recurrence and cancer-related death is high. Use of the term “palliative CRS/HIPEC” to describe the use of CRS/HIPEC in the setting of an incomplete cytoreduction in an asymptomatic patient should be considered a misnomer. While it might make sense in theory that there could be some therapeutic benefit (e.g., patients with less tumor burden may experience an improved symptom-free survival or respond better to systemic chemotherapy), at this time there is no data to show that patients who have an incomplete cytoreduction incur any survival benefit from the addition of HIPEC. Given the impact of CRS/HIPEC on QOL both acutely and over the long-term, (particularly in patients who are not symptomatic prior to the procedure), a patient who is without symptoms will certainly experience an acute, and potential chronic, loss of QOL. Is this cost reasonable for a theoretically improved QOL in the future?
It is essential for providers to keep this in mind when counseling patients who often see CRS/HIPEC as their only chance for meaningful survival. Many patients will be willing to accept a large impact on QOL for even a theoretical gain in survival. At this time, there are few absolute contraindications (extra-abdominal disease, extensive retroperitoneal involvement, infiltration of the root of the mesentery, poor performance status) to CRS/HIPEC (64). Each HIPEC surgeon decides for him or herself, based on the imperfect information of imaging studies and/or a diagnostic laparoscopy, and his or her own experience, whether or not to recommend CRS/HIPEC to a patient. Due to the nature of the goals of the profession, many surgical oncologists may not be comfortable with or have the time to provide the emotional and psychological support needed by the patient and family when CRS/HIPEC should not be recommended. By definition, all patients with peritoneal carcinomatosis have an advanced, life-threatening malignancy and are eligible for a palliative medicine consult-regardless of prognosis or the absence of physical symptoms. Early palliative medicine involvement has been shown to improve outcomes for patients and also provides help and support for the treating physicians in these challenging situations (44). At this time, a thorough work-up for CRS/HIPEC, curative or “palliative”, should include a baseline QOL assessment and a palliative medicine referral.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358-63. [PubMed]
- Franko J, Shi Q, Goldman CD, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol 2012;30:263-7. [PubMed]
- Tuthill M, Pell R, Guiliani R, et al. Peritoneal disease in breast cancer: a specific entity with an extremely poor prognosis. Eur J Cancer 2009;45:2146-9. [PubMed]
- Available online: www.nccn.org/professionals/physician_gls/pdf/palliativecare
- Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol 2012;30:880-7. [PubMed]
- Lambert LA. Looking up: Recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J Clin 2015;65:284-98. [PubMed]
- Spratt JS, Adcock RA, Muskovin M, et al. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res 1980;40:256-60. [PubMed]
- Dunlop PR, Hand JW, Dickinson RJ, et al. An assessment of local hyperthermia in clinical practice. Int J Hyperthermia 1986;2:39-50. [PubMed]
- Field SB, Morris CC. The relationship between heating time and temperature: its relevance to clinical hyperthermia. Radiother Oncol 1983;1:179-86. [PubMed]
- Leopold KA, Dewhirst M, Samulski T, et al. Relationships among tumor temperature, treatment time, and histopathological outcome using preoperative hyperthermia with radiation in soft tissue sarcomas. Int J Radiat Oncol Biol Phys 1992;22:989-98. [PubMed]
- Dewey WC. Interaction of heat with radiation and chemotherapy. Cancer Res 1984;44:4714s-20s. [PubMed]
- Urano M, Majima H, Miller R, et al. Cytotoxic effect of 1,3 bis (2-chloroethyl)-N-nitrosourea at elevated temperatures: Arrhenius plot analysis and tumour response. Int J Hyperthermia 1991;7:499-510. [PubMed]
- Gusani NJ, Cho SW, Colovos C, et al. Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann Surg Oncol 2008;15:754-63. [PubMed]
- Levine EA, Stewart JH 4th, Shen P, et al. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg 2014;218:573-85. [PubMed]
- Tabrizian P, Shrager B, Jibara G, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis: outcomes from a single tertiary institution. J Gastrointest Surg 2014;18:1024-31. [PubMed]
- Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009;27:6237-42. [PubMed]
- Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012;30:2449-56. [PubMed]
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128-37. [PubMed]
- Lerner B. Annals of Extreme Surgery. New York Times. 2011 August 29; Opinion.
- Pollock A. Hot Chemotherapy Bath: Patients See Hope, Critics Hold Doubts. New York Times. 2011 August 11; Business Day.
- González-Moreno S. Peritoneal Surface Oncology: A progress report. Eur J Surg Oncol 2006;32:593-6. [PubMed]
- Esquivel J, Elias D, Baratti D, et al. Consensus statement on the loco regional treatment of colorectal cancer with peritoneal dissemination. J Surg Oncol 2008;98:263-7. [PubMed]
- Helm JH, Miura JT, Glenn JA, et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Malignant Peritoneal Mesothelioma: A Systematic Review and Meta-analysis. Ann Surg Oncol 2015;22:1686-93. [PubMed]
- Miner TJ, Shia J, Jaques DP, et al. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg 2005;241:300-8. [PubMed]
- Feldman AL, Libutti SK, Pingpank JF, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol 2003;21:4560-7. [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [PubMed]
- Baratti D, Kusamura S, Iusco D, et al. Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: a two-center study of 101 patients. Dis Colon Rectum 2014;57:858-68. [PubMed]
- Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 2009;27:681-5. [PubMed]
- Hill AR, McQuellon RP, Russell GB, et al. Survival and quality of life following cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colonic origin. Ann Surg Oncol 2011;18:3673-9. [PubMed]
- Ba MC, Cui SZ, Lin SQ, et al. Chemotherapy with laparoscope-assisted continuous circulatory hyperthermic intraperitoneal perfusion for malignant ascites. World J Gastroenterol 2010;16:1901-7. [PubMed]
- McQuellon RP, Loggie BW, Fleming RA, et al. Quality of life after intraperitoneal hyperthermic chemotherapy (IPHC) for peritoneal carcinomatosis. Eur J Surg Oncol 2001;27:65-73. [PubMed]
- Tan WJ, Wong JF, Chia CS, et al. Quality of life after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: an Asian perspective. Ann Surg Oncol 2013;20:4219-23. [PubMed]
- Esquivel J, Piso P, Verwaal V, et al. American Society of peritoneal surface malignancies opinion statement on defining expectations from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with colorectal cancer. J Surg Oncol 2014;110:777-8. [PubMed]
- Chia CS, Tan WJ, Wong JF, et al. Quality of life in patients with peritoneal surface malignancies after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol 2014;40:909-16. [PubMed]
- Duckworth KE, McQuellon RP, Russell GB, et al. Patient rated outcomes and survivorship following cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy (CS + HIPEC). J Surg Oncol 2012;106:376-80. [PubMed]
- Miner TJ. Palliative surgery for advanced cancer: lessons learned in patient selection and outcome assessment. Am J Clin Oncol 2005;28:411-4. [PubMed]
- de Mestier L, Volet J, Scaglia E, et al. Is palliative laparoscopic hyperthermic intraperitoneal chemotherapy effective in patients with malignant hemorrhagic ascites? Case Rep Gastroenterol 2012;6:166-70. [PubMed]
- Facchiano E, Scaringi S, Kianmanesh R, et al. Laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) for the treatment of malignant ascites secondary to unresectable peritoneal carcinomatosis from advanced gastric cancer. Eur J Surg Oncol 2008;34:154-8. [PubMed]
- Garofalo A, Valle M, Garcia J, et al. Laparoscopic intraperitoneal hyperthermic chemotherapy for palliation of debilitating malignant ascites. Eur J Surg Oncol 2006;32:682-5. [PubMed]
- Patriti A, Cavazzoni E, Graziosi L, et al. Successful palliation of malignant ascites from peritoneal mesothelioma by laparoscopic intraperitoneal hyperthermic chemotherapy. Surg Laparosc Endosc Percutan Tech 2008;18:426-8. [PubMed]
- Randle RW, Swett KR, Swords DS, et al. Efficacy of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in the management of malignant ascites. Ann Surg Oncol 2014;21:1474-9. [PubMed]
- Valle M, Van der Speeten K, Garofalo A. Laparoscopic hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) in the management of refractory malignant ascites: A multi-institutional retrospective analysis in 52 patients. J Surg Oncol 2009;100:331-4. [PubMed]
- Ba MC, Long H, Cui SZ, et al. Multivariate comparison of B-ultrasound guided and laparoscopic continuous circulatory hyperthermic intraperitoneal perfusion chemotherapy for malignant ascites. Surg Endosc 2013;27:2735-43. [PubMed]
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. [PubMed]
- Albertsmeier M, Hauer A, Niess H, et al. Quality of life in peritoneal carcinomatosis: a prospective study in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Dig Surg 2014;31:334-40. [PubMed]
- Alexander HR, Mavroukakis S, Libutti S, et al. Impact of tumor resection (Rxn) and intraperitoneal (IP) chemotherapy (CHRx) on health related quality of life (HRQL) in patients (Pts) with peritoneal surface malignancies (PSM). Ann Surg Oncol 2004;11.
- Alves S, Mohamed F, Yadegarfar G, et al. Prospective longitudinal study of quality of life following cytoreductive surgery and intraperitoneal chemotherapy for pseudomyxoma peritonei. Eur J Surg Oncol 2010;36:1156-61. [PubMed]
- Argenta PA, Sueblinvong T, Geller MA, et al. Hyperthermic intraperitoneal chemotherapy with carboplatin for optimally-cytoreduced, recurrent, platinum-sensitive ovarian carcinoma: a pilot study. Gynecol Oncol 2013;129:81-5. [PubMed]
- Jess P, Iversen LH, Nielsen MB, et al. Quality of life after cytoreductive surgery plus early intraperitoneal postoperative chemotherapy for pseudomyxoma peritonei: a prospective study. Dis Colon Rectum 2008;51:868-74. [PubMed]
- Kirby R, Liauw W, Zhao J, et al. Quality of life study following cytoreductive surgery and intraperitoneal chemotherapy for pseudomyxoma peritonei including redo procedures. Int J Surg Oncol 2013;2013:461041.
- Lim C, Tordjmann D, Gornet JM, et al. Prospective study of quality of life after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy using oxaliplatin for peritoneal carcinomatosis. Bulletin du Cancer 2010;97:1053-60. [PubMed]
- Lim SJ, Cormier JN, Feig BW, et al. Toxicity and outcomes associated with surgical cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC) for patients with sarcomatosis. Ann Surg Oncol 2007;14:2309-18. [PubMed]
- Macrì A, Maugeri I, Trimarchi G, et al. Evaluation of quality of life of patients submitted to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinosis of gastrointestinal and ovarian origin and identification of factors influencing outcome. In Vivo 2009;23:147-50. [PubMed]
- McQuellon RP, Danhauer SC, Russell GB, et al. Monitoring health outcomes following cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol 2007;14:1105-13. [PubMed]
- McQuellon RP, Loggie BW, Lehman AB, et al. Long-term survivorship and quality of life after cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol 2003;10:155-62. [PubMed]
- McQuellon RP, Russell GB, Shen P, et al. Survival and health outcomes after cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for disseminated peritoneal cancer of appendiceal origin. Ann Surg Oncol 2008;15:125-33. [PubMed]
- Passot G, Bakrin N, Roux AS, et al. Quality of life after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy: a prospective study of 216 patients. Eur J Surg Oncol 2014;40:529-35. [PubMed]
- Schmidt U, Dahlke MH, Klempnauer J, et al. Perioperative morbidity and quality of life in long-term survivors following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol 2005;31:53-8. [PubMed]
- Tsilimparis N, Bockelmann C, Raue W, et al. Quality of life in patients after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is it worth the risk? Ann Surg Oncol 2013;20:226-32. [PubMed]
- Tuttle TM, Zhang Y, Greeno E, et al. Toxicity and quality of life after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2006;13:1627-32. [PubMed]
- Zenasni F, Botella M, Elias D, et al. The long-term impact of hyperthermic intraperitoneal chemotherapy on survivors treated for peritoneal carcinomatosis: a cross-sectional study. Support Care Cancer 2009;17:1255-61. [PubMed]
- McQuellon R, Gavazzi C, Piso P, et al. Quality of life and nutritional assessment in peritoneal surface malignancy (PSM): recommendations for care. J Surg Oncol 2008;98:300-5. [PubMed]
- Sloan JA, Frost MH, Berzon R, et al. The clinical significance of quality of life assessments in oncology: a summary for clinicians. Support Care Cancer 2006;14:988-98. [PubMed]
- Brücher BL, Piso P, Verwaal V, et al. Peritoneal carcinomatosis: cytoreductive surgery and HIPEC--overview and basics. Cancer Invest 2012;30:209-24. [PubMed]
- Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 2010;28:63-8. [PubMed]
- Elias D, Goere D, editors. Advances in Peritoneal Surface Oncology. Vol 169. Germany: Springer-Verlag, 2010.


