Metastatic colon cancer from extrahepatic cholangiocarcinoma presenting as painless jaundice: case report and literature review
Background
Cholangiocarcinoma (CCA) is a rare, but lethal cancer arising anywhere from the intrahepatic to the extrahepatic biliary epithelium. It represents about 10% of all primary hepatobiliary malignancies and accounts for approximately 3% of all gastrointestinal cancers. CCA arises from the biliary epithelium and may occur anywhere along the biliary tree. About two thirds are located at the hepatic duct confluence (hilum) and the remaining one third occurring within the intrahepatic biliary and distal common bile duct epithelium. Adenocarcinoma comprises approximately 95% of all subtypes (1). Regardless of the site of origin, CCA is a highly aggressive malignancy and confers a dismal prognosis with majority of patients presenting with advanced unresectable disease. The most common mode of distant spread is via the lymphatics with the lungs, adrenal glands, and brain being the most common extra-hepatobiliary areas of spread. However, metastatic CCA of the colorectum is rare with only sporadic case reports of intrahepatic CCA described in the surgical literature. We present a case of incidental synchronous metastatic colonic adenocarcinoma in a patient with primary extra-hepatic CCA with review of the surgical literature.
Case presentation
A 61-year-old Caucasian female presented with two-month history of painless jaundice associated with worsening pruritus, malaise, anorexia, and over 30 pound unintentional weight loss. She had jaundice, but afebrile and benign abdominal exam. Laboratory data was remarkable for a total bilirubin, 26 mg/dL; alkaline phosphatase (ALP), 333 IU/L; CEA, 3.6 ng/mL; CA19-9, 21 U/mL; and white blood cell count (WBC), 22.9×109/L.
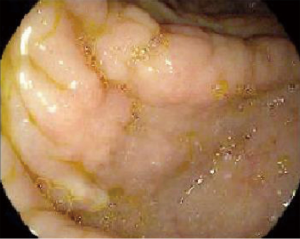
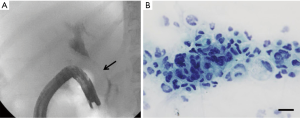
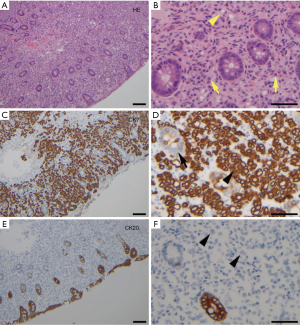
A contrast-enhanced CT of the abdomen showed proximal and intrahepatic bile ducts prominence without focal intrahepatic or biliary mass lesions. Incidentally, there was diffuse thickening of the proximal colon without any focal mass lesions or obstruction. The patient underwent an endoscopic retrograde cholangiopancreatography (ERCP) that showed a long 7 cm high-grade stricture extending from the distal common bile duct to the common hepatic duct with proximal common hepatic and intrahepatic ductal dilatation (Figure 1A). Multiple brushings were obtained and a 10 Fr ×9 cm plastic stent placed for drainage. The patient had symptomatic improvement and her total bilirubin stabilized at 15 mg/dL. Brushing cytology confirmed malignant cells consistent with CCA (Figure 1B). A diagnostic colonoscopy obtained by primary service to evaluate diarrhea showed multiple discrete, hard submucosal nodularities throughout the transverse and ascending colon (Figure 2). Biopsies were obtained and final pathology was consistent with poorly differentiated adenocarcinoma and immunohistochemistry confirmed cytokeratin 7 (CK-7) positivity and cytokeratin 20 (CK-20) negativity (Figure 3A-F); findings consistent with a synchronous CCA metastasis to the colon. The patient deteriorated and elected for hospice and medical palliative care.
Discussion
Most hepatic malignancies are metastatic in origin with GI metastases being the most common primary accorded by the rich portal circulation. However, metastatic GI malignancy from hepatobiliary cancer including CCA is extremely rare. CCA often spreads by contiguous perineural, vascular and peritoneal invasion as well as lymphatic dissemination. Common sites of distant metastasis include liver, lungs, brain, adrenal glands, and the spine (2). Very rarely however, has CCA metastasis to the colon been described. The mode of such spread is poorly understood. Only five cases have ever been reported in the literature describing this rare pattern (Table 1) (3-7). Analyzing and extrapolating from these limited case reports, it is highly unlikely that lymphatic or peritoneal dissemination are the mode of colonic spread as there were no evidence of peritoneal seeding at exploration or any lymph node involvement from oncologic resection. Therefore, hematogenous spread offers the most plausible mode of metastasis. This is consistent with the mode of spread of metastatic colorectal cancer from other primaries such as breast, stomach, lung, prostate, and gallbladder (8,9).

Full table
In a patient presenting with a colonic mass and a concurrent history of CCA, distinguishing primary from metastatic or secondary colorectal cancer not only has prognostic ramifications, but affects management options and outcomes. As mentioned previously, metastatic liver cancer from colorectal primary is the most common cause of hepatic malignancy. In selected group of patients, hepatic metastasectomy improves survival. However, secondary colorectal cancer is often a telltale sign of unresectability.
Based on unique trends in our case report and the limited similar cases in the literature, we have identified key features that may distinguish primary colon cancer from CCA metastases to the colon. These features may be classified into endoscopic, clinicopathologic, and immunohistochemical characteristics. Endoscopically, as illustrated in our case report, the colonic lesions consist of multiple discrete submucosal nodularity with intact mucosa. The submucosal infiltration by metastatic cancer often results in stricture formation. This is unlike primary colon cancer, which is often focal, bleeding ulceration, loss of lobulation, and originating from the mucosa with radial extension (10). Histopathological features often demonstrate glandular epithelium consistent with adenocarcinoma similar to CCA. However, the mucosa of the colonic metastatic tumor is often preserved.
Immunohistochemical staining with CK-7 and CK-20 is the most reliable feature for distinguishing primary colonic adenocarcinoma from metastatic CCA to the colon. This may also be used to differentiate intrahepatic CCA and metastatic liver tumor from primary colorectal cancer. Rullier and colleagues, in a retrospective study, evaluated CK-7 and CK-20 expression in CCA arising from site of the biliary tree to determine the distinction between CCA and hepatic metastasis from primary colorectal carcinoma. CK-20 is positive in approximately 70-95% of colorectal and only 20-40% of pancreaticobiliary adenocarcinomas, whereas CK-7 is positive in 90-100% of pancreaticobiliary and only 5-25% of colorectal adenocarcinomas. They concluded that CK7+, CK20− tumors have approximately a 100% positive predictive value for establishing the diagnosis of primary and metastatic CCA to other sites including the colorectal areas. Whereas CK7−, CK20+ was equally highly predictive of hepatic metastasis from a primary colorectal carcinoma (11). Our patient’s immunophenotype was highly consistent with CK7+, CK20−; confirming that the colonic adenocarcinoma was not a second primary, but a metastatic tumor from the extrabiliary CCA.
The management of CCA metastases or local invasion into the colon remains to be defined. Whether screening colonoscopy and the extent of colonic resection in patients with CCA metastasis to colon affect morbidity, overall survival, or quality of life remains to be elucidated. Three of the five reported cases underwent palliative colonic resection with two patients presenting with colonic metastatic lesions at least five years after index hepatectomy for CCA. One patient underwent curative resection of the colonic lesion with long-term survival observed. Therefore, in patients with resectable CCA and no evidence of colonic or peritoneal visceral involvement colonoscopic evaluation prior to embarking into any major hepatobiliary surgery may influence management strategy. Moreover, whether survivors of CCA should undergo shorter interval screening colonoscopy compared to the general population to evaluate for distant recurrence may be worth addressing.
Conclusions
Metastatic CCA to the colon is a rare pattern of distant spread that may pose a diagnostic challenge. Although the diagnosis may be a difficult process, it is imperative to establish the difference between primary colonic cancer and metastatic colon cancer as this may impact surgical management, prognosis, and possibly quality of life. In this case report and extensive review of the limited cases reported, we have identified some salient characteristics that may assist in the differentiation of primary colon cancer and metastatic colon cancer from CCA. Nevertheless, little remains known about the pathogenic behavior of metastatic secondary colorectal cancer. And more so, the management approach to such metastatic cancer still remains to be defined. Screening colonoscopy in patients presenting with resectable CCA may alter management. Furthermore, whether patients with history of resected CCA may benefit from a more frequent screening colonoscopy remains to be validated.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kambakamba P, DeOliveira M. Perihilar cholangiocarcinoma: paradigms of surgical management. Am J Surg 2014;208:563-70. [PubMed]
- Lee YT, Geer DA. Primary liver cancer: pattern of metastasis. J Surg Oncol 1987;36:26-31. [PubMed]
- Tokodai K, Kawagishi N, Miyagi S, et al. Intestinal obstruction caused by colonic metastasis from intrahepatic cholangiocarcinoma 6 years after removal of the primary tumor: report of a case. Surg Today 2012;42:797-800. [PubMed]
- Mimatsu K, Oida T, Kawasaki A, et al. Long-term survival after resection of mass-forming type intrahepatic cholangiocarcinoma directly infiltrating the transverse colon and sequential brain metastasis: Report of a case. Surg Today 2011;41:1410-3. [PubMed]
- Fujii K, Goto A, Yoshida Y, et al. Gastrointestinal: Transmural colonic metastasis arising from primary cholangiocarcinoma. J Gastroenterol Hepatol 2010;25:1329. [PubMed]
- Izzo F, Piccirillo M, Albino V, et al. Case report: appearance of an intestinal metastasis from intrahepatic cholangiocarcinoma occurring 5 years after resection of the primary tumor. Eur J Gastroenterol Hepatol 2010;22:892-4. [PubMed]
- Wakahara T, Tsukamoto T, Kitamura S, et al. Metastatic colon cancer from intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg 2005;12:415-8. [PubMed]
- Balthazar EJ, Rosenberg HD, Davidian MM. Primary and metastatic scirrhous carcinnoma of the rectum. AJR Am J Roentgenol 1979;132:711-5. [PubMed]
- Ota H, Azekura K, Seki M, et al. Clinico pathological study on metastatic colorectal cancer. Stomach Intestine 1988;23:633-43.
- Hong SP. Malignant Tumors in Colon. In: Chun JH, Yang KS, Choi MG, editors. Clinical Gastrointestinal Endoscopy: A Comprehensive Atlas. New York: Springer Dordrecht Heidelberg London New York, 2014:475-98.
- Rullier A, Le Bail B, Fawaz R, et al. Cytokeratin 7 and 20 expression in cholangiocarcinomas varies along the biliary tract but still differs from that in colorectal carcinoma metastasis. Am J Surg Pathol 2000;24:870-6. [PubMed]