Surgical technology and pharmacology of hyperthermic perioperative chemotherapy
Introduction
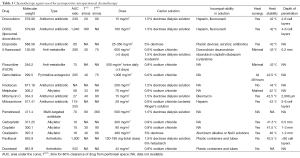
Cytoreductive surgery (CRS) and hyperthermic perioperative chemotherapy (HIPEC) have evolved over three decades and is now a standard of care for peritoneal metastases from appendiceal epithelial cancers, colorectal cancer and peritoneal mesothelioma (1,2). Promising results for HIPEC in recurrent ovarian cancer have been published (3,4) as a result of continued research efforts by dedicated investigations in the management of peritoneal metastases (5-8). Multiple variables that have an effect on outcome have been identified. There is a near universal opinion regarding the surgery in that all data shows that the more complete the cytoreduction, the greater the benefits that will occur from this combined treatment (9-12). For long-term benefit with gastrointestinal peritoneal metastases, removal of disease to a microscopic or near-microscopic extent is required (13,14). For cytoreduction of ovarian cancer, peritoneal dissemination or malignant peritoneal mesothelioma, the resection of abdominal and pelvic disease should be as complete as is possible (15,16). However, it is obvious from a survey of the literature that no standardized perioperative chemotherapy treatment currently exists. Table 1 identifies five patient-related variables for CRS and HIPEC, ten methodological variables for HIPEC and itemizes the use of pharmacologic variables for chemotherapy agents that are currently available for administration in the operating room as HIPEC (17) or in the early postoperative period as early postoperative intraperitoneal chemotherapy (EPIC) (18). Over 30 variables are listed as potential differences for the application of CRS and HIPEC. Randomized clinical trials adequately powered to answer important questions concerning optimal treatments are not likely to be completed in a timely manner. There are some important clinical studies that would select the most important differences in treatment (19,20). However, no comprehensive answers will soon be available. For this reason, this review seeks to establish the important theoretical considerations for an optimal HIPEC. The goals of this manuscript are to establish the requirements for perioperative chemotherapy delivery and suggest optimal treatment strategies that need to be incorporated into the management plan of all patients.

Full table
Prior to HIPEC and/or EPIC the abdomen and pelvis are to be cleared of all visible tumor masses and peritoneal nodules. This requires knowledgeable patient selection to avoid morbidity and mortality and allow complete tumor removal. A series of peritonectomy procedures includes right upper quadrant peritonectomy, left upper quadrant peritonectomy, pelvic peritonectomy, lesser omentectomy with omental bursectomy, and anterior parietal peritonectomy. Normal appearing peritoneal surfaces are not stripped. Visceral resections include greater omentectomy-splenectomy, right colectomy, rectosigmoid colon resection and occasionally partial gastrectomy. Again, only structures coated by disease are resected. HIPEC follows the CRS and usually precedes bowel reconstruction and closure of the abdomen.
The important variables for presentation are listed in Table 2. (I) A proper selection of chemotherapy agents is required; (II) the proper duration of HIPEC as part of the combined treatment for peritoneal metastases is necessary; (III) the level of heat for hyperthermia appropriate for a particular chemotherapy agent is required; (IV) there are several different techniques for abdominal irrigation prior to HIPEC that can be considered; (V) five different methodologies for HIPEC exist; (VI) there are ten different companies that commercially sell hyperthermia pumps; (VII) an important aspect of HIPEC by the open technologies is the commercially available table-mounted retractors; (VIII) finally, laparoscopic HIPEC in selected patients will be described.

Full table
Proper selection of chemotherapy agents for HIPEC
Perhaps the most crucial aspect of an optimal HIPEC is the selection of a chemotherapy agent and its proper dose for use within the peritoneal space. To select a chemotherapy agent one must know the response expected with this drug in patients with metastatic disease. The area under the curve (AUC) ratio is important in that it estimates the dose intensity expected in the treatment of peritoneal metastases as compared to the toxicity experienced as a result of systemic effects of the drug.
Those drugs that are used in the operating room with heat are acute phase drugs that can exert their effects in the absence of cell proliferation (17). Those drugs that are used for EPIC are selected because they are not augmented by heat and they require cell division for their optimal effects. Such drugs are 5-fluorouracil and paclitaxel (15,17). Bakrin and colleagues presented data suggesting that the combination of hyperthermia with a drug shown to have developed systemic drug resistance may be effective with hyperthermia when used within the peritoneal space (3). These data showed that cisplatin-resistant ovarian cancer patients had the same benefits from CRS and HIPEC with cisplatin as the group of patients who were judged to be cisplatin-sensitive.
The AUC ratio of an intraperitoneal chemotherapy agent estimates the exposure of peritoneal metastases to drug as compared to the exposure of the body compartment. As shown in Table 3, many of the drugs selected for HIPEC have a respectable AUC ratio (21). The heat-augmented drugs with the most favorable AUC ratios are mitomycin C, doxorubicin, gemcitabine, and pegylated-liposomal doxorubicin.
Full table
The retention of the intraperitoneal chemotherapy agent is crucial in drug selection in that a response of the peritoneal metastasis is dependent upon the time over which a particular concentration of drug is present at the surface of the nodule. Slow clearance of the intraperitoneal drug and prolonged hyperthermia would be expected to cause a maximal response. Heat-augmented drugs which have a prolonged retention are gemcitabine and pegylated-liposomal doxorubicin.
Another strategy for prolonged exposure of peritoneal nodules to chemotherapy comes by continuous infusion of a heat-augmented drug. The best studied intravenous chemotherapy agent targeted to heated peritoneal surfaces is ifosfamide. Continuous infusion of ifosfamide during HIPEC will result in cytotoxic levels of this drug within the peritoneal nodule over the 90 minutes of HIPEC (22). Also, 5-fluorouracil has been used as a bolus infusion to augment the effects of hyperthermic intraperitoneal oxaliplatin (23).
A third mechanism for increased drug retention within the peritoneal space during HIPEC is repeated dosing of the chemotherapy agents. Verwaal and colleagues used a triple dosing schedule for mitomycin C in order to increase the intraperitoneal exposure of this drug. They used half the drug dose at the initiation of HIPEC, one-quarter of it at 30 minutes, and another one-quarter of the dose at 60 minutes for a total of 90 minute HIPEC. By their calculations, this increased the effective dose of the mitomycin C (6).
Rationale for a combined treatment for peritoneal metastases utilizing CRS plus HIPEC
The treatment of peritoneal metastases from gastrointestinal or ovarian cancer was never reported as successful if CRS alone or intraperitoneal chemotherapy alone was used as an isolated treatment. Success was first recognized when the CRS with peritonectomy was combined with perioperative hyperthermic chemotherapy as a planned surgical procedure (24). In resecting abdominal or pelvic deposits of cancer in a patient with known peritoneal metastases, contamination of the dissected surfaces is unavoidable. This combination of cancer surgery and resection site plus peritoneal progression of disease has been called “tumor cell entrapment” (25). An opportunity to interrupt this inevitable contamination of the surgical resection sites with cancer implants requires the eradication of these implants prior to their entrapment within fibrinous material and scar tissue that is part of the inflammatory process. Sugarbaker et al. hypothesized that attempts to eliminate cancer cells from peritoneal surfaces were limited to intraoperative events or chemotherapy lavage limited to the first 5 postoperative days (18). These treatments would then occur before fibrosis sets in as a part of the healing of the surfaces of the abdomen and pelvis. From a theoretical perspective, in order to prevent entrapment of cancer cells within suture lines or within the abdominal incision, the chemotherapy solutions must be used in the operating room after the resections but prior to the performance of an intestinal anastomosis, and prior to the closing of the abdominal wall.
Not only are patients who have surgery for peritoneal metastases at risk for tumor cell entrapment. Following a potentially curative resection of a pancreas cancer, disease recurrence has been documented in the local and regional area in 50% of patients and on peritoneal surfaces in 40-60% of patients (26). Similarly, in gastric cancer patients who fail the surgical resection of the primary disease, 54% will progress with peritoneal metastases (27). In colorectal cancer, the local and regional failure rate is less common but still exists in approximately 30% of those patients who fail surgical treatment (28). Currently, protocols exist attempting to use HIPEC to reduce or eradicate local-regional failures in those patients who are at risk for subsequent local failure and/or peritoneal metastases. The COLOPEC trial is currently active in the Netherlands and the PROMENADE protocol is in the process of being activated from Rome, Italy (29,30). In these primary malignancies cancer cells disseminated as a result of the trauma of the surgical resection must be eliminated from the abdominal and pelvic space prior to the onset of adhesions and the healing process.
Timing with HIPEC is also important for the duration of the treatment. The hyperthermia alone does not bring about a mass destruction of peritoneal metastases. The cytotoxicity of the treatment comes from the simultaneous use of cancer chemotherapy and heat. The heat significantly increases the cytotoxicity of a limited number of chemotherapy agents (31). This means that the hyperthermia should be applied while the chemotherapy is present within the peritoneal space but need not be continued as a treatment in and of itself. Knowledge of the proper length of time for HIPEC depends on the pharmacologic parameters established for the intraperitoneal administration of the chemotherapy agent.
In Table 3, the chemotherapy agents frequently used for HIPEC are listed. Also shown is the intraperitoneal half-life, the time at which 80% of the drug has cleared from the peritoneal space and the AUC of peritoneal concentration times time divided by the intravenous concentration times time. We can see that one of the most rapidly cleared drugs is oxaliplatin. Its t½ within the peritoneal space is approximately 40 minutes and 80% of the drug leaves the peritoneal space within 60 minutes. Consequently, the duration of hyperthermia for intraperitoneal oxaliplatin is 30 minutes (32). For mitomycin C the t½ is 40 minutes and 80% of the drug is gone from the peritoneal space within 90 minutes. Usually, the duration of HIPEC for mitomycin C is 90 minutes (33). Similar pharmacologic parameters exist for doxorubicin. The t½ was 12 minutes. At 90 minutes 80% of the drug is cleared from the peritoneal space (34). For liposomal doxorubicin, there is a profound retention of drug within the peritoneal space. Pegylated liposomal doxorubicin is a nanoparticle with a large molecular size as compared to free doxorubicin. The t½ for pegylated liposomal doxorubicin is estimated at 180 minutes. The time for 80% clearance has not been determined. The duration of HIPEC when pegylated liposomal doxorubicin is used is 3 hours.
Level of hyperthermia
Adding hyperthermia to intraperitoneal chemotherapy will increase the tumor response by several mechanisms. First, heat alone has some direct anti-tumor effects. Although potentially important, the extent of the temperature elevation within the core of a tumor nodule is extremely limited. Selective cytotoxicity of malignant cells by heat is related to impaired DNA repair, increased protein denaturation, increased acidity, lysosomal activation, and increased apoptotic cell death (35).
A second and perhaps more important augmentation of hyperthermia is increased cytotoxicity with heat. Synergy between heat and cancer chemotherapy drugs is a complex pharmacologic event. Augmented effects have been demonstrated for doxorubicin, cisplatin, mitomycin C, melphalan, oxaliplatin, and gemcitabine (36).
A third mechanism for increased peritoneal metastases cell kill with hyperthermia is related to increased depth of penetration of the cancer chemotherapy into tumor nodules. Jacquet et al. reported increased tissue penetration of doxorubicin when the cancer chemotherapy solution was administered intraperitoneally at 43 °C. This increase in tissue concentration did not affect the pharmacokinetic advantages of the intraperitoneal administration (37). The elevated interstitial fluid pressure in tumor nodules compared to normal tissue is an acknowledged phenomenon (38). A thermal dose-dependent decrease in interstitial fluid pressure in experimental solid tumors in an animal model have been reported by Leunig et al. (39).
However, the level of hyperthermia must be matched to the intraperitoneal cancer chemotherapy agent. With cisplatin, the higher the temperature, the greater the increase in cytotoxicity. In addition, those chemotherapy agents that function as pro-drugs may have a temperature threshold for maximal augmentation of cytotoxicity. Mitomycin C and Gemzar are included in this category. It has been shown that Gemzar with 43 °C heat is impaired in its cytotoxicity. It is postulated that the conversion of gemcitabine triphosphate (the active agent) may be inhibited intracellularly with high heat. Therefore, with this drug intraperitoneal heat should be limited to 41-42 °C (40). The same situation is likely to exist with mitomycin C.
Urano and colleagues identified the cancer chemotherapy agents that are augmented by moderate hyperthermia of 41 °C. The drugs most increased in their cytotoxicity were cisplatin, melphalan, ifosfamide, and cyclophosphamide (31). These “super drugs” for hyperthermia are not all appropriate for intraperitoneal administration. Ifosfamide and cyclophosphamide are pro-drugs which are expected to show little cytotoxicity when present with cancer cells in a chemotherapy solution. However, cisplatin and melphalan should enter the peritoneal metastases well, be augmented by 43-44 °C hyperthermia with a marked therapeutic effect expected.
Irrigation techniques
Although HIPEC has received the greatest attention for eradication of the cellular component of peritoneal metastases following CRS, other mechanisms may be of value or less toxic. The mechanical removal of cancer cells through intraoperative irrigation prior to HIPEC may help assist in the maximal eradication of cancer cells. No doubt that in performing CRS for peritoneal metastases, large numbers of cancer cells will be present within the ascites fluid, will be disrupted from the peritonectomy specimens, or released from traumatized tumor nodules. Frequently throughout the CRS, dissection sites should be irrigated copiously and thoroughly aspirated. This frequent irrigation is to remove blood, tissue debris, and stray cancer cells as well as to clarify the anatomy for safe subsequent dissection. As a parietal peritonectomy procedure is completed, a large volume of warm saline irrigation should flood the peritonectomy site and the fluid should be vigorously manipulated to remove biologic fluids and cells. After the complete removal of the irrigation fluid, laparotomy pads or sterile towels should be placed in the peritonectomy site to prevent cancer cells from implanting within the raw surfaces as additional cytoreduction proceeds (25).
Upon completion of the cytoreduction and prior to HIPEC, an irrigation with a cytotoxic but non-chemotherapeutic agent should occur. Peroxide at 0.24% in 3 L of warm saline is used at the MedStar Washington Hospital Center (41). Others have used 3 L of warm distilled water. Still others have utilized a dilute povidone-iodine solution (42).
Kuramoto and colleagues have shown the value of mechanical cleansing of the peritoneal space with a large volume of fluid. They have used extensive intraperitoneal lavage (EIPL) to improve the survival of patients with gastric cancer and a high risk for implantation of gastric cancer cells (43). His strategy is to use 10 L of warm saline, 1liter at a time in order to maximally irrigate away cancer cells that may be present.
Technologies for HIPEC
As might be expected, several different methodologies for administering HIPEC have been developed at centers experienced in the management of peritoneal surface malignancy. The open technique with a vapor barrier created by smoke evacuators has been used extensively at the MedStar Washington Hospital Center (Figure 1) (44). An open coliseum technique is with the abdomen covered by a plastic sheet and access for manipulation of the intraabdominal contents obtained by a cruciate incision within the plastic cover (Figure 2) (45). A closed technique that has open access has been described by Rat and colleagues and is referred to as the Landager technique (Figure 3) (46). Some groups close the abdomen prior to the HIPEC administration and then following HIPEC open the abdomen to perform anastomoses, repair seromuscular tears, and then close the abdominal incision. In this closed technique the skin only is closed in a watertight fashion so that all of the structures of the anterior abdominal wall are thoroughly treated by the chemotherapy solution. Finally, some use a totally closed technique. In this methodology the CRS is performed, the abdomen is irrigated prior to the performance of intestinal anastomoses and the closure of the abdominal incision. Tubes and drains are positioned prior to the definitive closure of the abdomen. The cancer chemotherapy is then administered in the operating room prior to the patient being taken to the surgical intensive care unit.
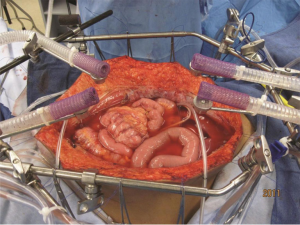
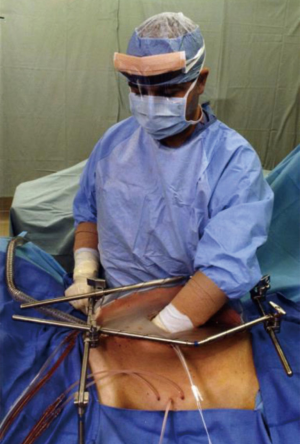
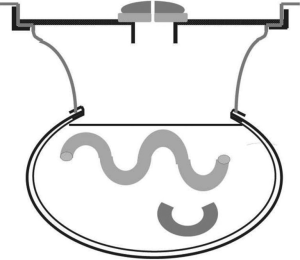
Table 4 itemizes the credits and debits of the open versus closed abdomen technique.

Full table
The safety of the open technique has been repeatedly demonstrated in that at the levels of detection possible, no chemotherapy aerosols have been found to be present within the operating room environment. However, from a theoretical perspective, some drugs should definitely be used only by the closed technique. One of these drugs is melphalan. Melphalan is nitrogen mustard and is an aromatic compound which may, with moderate heat, escape into the operating room environment. Melphalan has been recommended only for use using a closed technique.
Commercially available hyperthermia pumps
To date, ten different commercial groups are manufacturing hyperthermia pumps (Table 5). The list of the hyperthermia pumps, the location of their production, and their approval for EC (European Community) or for US FDA (US Food and Drug Administration) is also indicated. The Braile apparatus is only approved for use in Brazil.
Full table
All of the devices can effectively heat the intraperitoneal fluid to 44 °C. All of them are monitored at several sites within the abdomen and pelvis with thermister probes. There are variable maximal rates of flow which will influence the rate at which the intraperitoneal fluid can be heated to the desired 42 °C temperature. Some of the apparatus may be more appropriate for open administration (Belmont, SunChip, Euromedical). Others are more appropriate for the closed system (RanD Performer, Hyperthermic Solutions, Cavitherm).
Approximately 20% of the centers in the US performing HIPEC still use a “homemade” machine. Oftentimes this is a cardiopulmonary bypass machine with a water bath at the inflow so that the desired temperatures can be reached within the peritoneal space. Inflow temperatures need to be 45-46 °C in order to, within a reasonable time period, reach appropriate temperatures within the peritoneal space.
Commercially available table-mounted retractors
In order to perform the open technique, the skin edges are elevated on the frame of a retractor that is mounted to the operating table. Figure 1 shows the Thompson retractor with skin edges elevated with the abdominal incision held open with the fixed retractors (Thompson Surgical Instruments, Traverse City, MI, USA). A Bookwalter retractor can give the same exposure (Bookwalter Retractor, Symmetry Surgical, Antioch, TN, USA). Also, the Omni retractor has been used for this purpose (Omni Retractor, Omni-Tract Surgical, St. Paul, MN, USA).
Role of laparoscopy in peritoneal metastases patients
In the last two decades laparoscopy has been explored as a tool in both diagnosis, staging and treatment of patients with peritoneal metastases.
Laparoscopy in diagnosis and staging of peritoneal metastases
It is a well-established fact that medical imaging techniques underscore the extent and location of peritoneal metastases. Neither does it provide a histological diagnosis. In a recent evaluation by Pasqual et al. preoperative CT and FDG-PET/CT failed to detect PC in 9% and 17% of cases (47). The potential advantages of laparoscopy in the diagnostic-staging process are obvious: direct visualization of the peritoneal disease and its extent, accurate evaluation of serosal surface of the small bowel, opportunity to take multiple biopsies. Several scoring systems assessing the extent and localization of peritoneal metastases have been developed. They are important proxies for the probability of achieving a complete cytoreduction which is the most important prognostic variable for survival. Three early single center studies suggested that laparoscopy was useful in scoring the extent of peritoneal metastases of mesothelioma, colorectal and appendiceal cancer (48-50). All however had small sample size and lacked application of the laparoscopy in a consecutive manner. Since, new data have emerged. In a multicentric trial (Olympia-MITO 13), staging laparoscopy reached an over 80% accuracy in assessing peritoneal spread in ovarian cancer peritoneal metastases (51). Valle et al. reported on staging laparoscopy in 351 patients with peritoneal metastases and documented a 1.42% understaging rate in this series (52). Some areas such as the lesser sac present a higher risk for inadequate staging during laparoscopy. In a large retrospective analysis of 6,687 patients undergoing colorectal resection Thomassen et al. reported that peritoneal metastases were detected in 1.4% of patients undergoing laparoscopic resection and 5% of patients undergoing open resection, and this after adjustment for patient and tumor characteristics (53). Several attempts have been made to increase the accuracy of staging laparoscopy by using hand assisted techniques or enhanced imaging modes (fluorescence) (54,55).
Laparoscopy in treatment of peritoneal metastases
The emerging role of laparoscopy in the treatment of peritoneal metastases has been initiated by advancements in both laparoscopy itself and the understanding of natural evolution of peritoneal metastases.
First, over the years laparoscopy has established itself as a valid surgical tool in surgical oncology. Prospective randomized trials have demonstrated no difference in distant parenchymateous metastases, incisional recurrences and disease-free and overall survival in patients undergoing laparoscopic resection of their intra-abdominal malignancy if adequate precautions are taken (56,57). Some studies even suggest a better outcome for laparoscopically resected primary colon cancer. The underlying rationale is that laparoscopic surgery is not a different surgery, merely a different surgical approach.
Second, more patients with peritoneal metastases are presented to the surgical team earlier in their course of disease. This more limited disease may be more open to the laparoscopic approach. CRS and HIPEC are clearly moving up in the timeline of treatment for these patients. Alternatively a proactive application of intraperitoneal chemotherapy is currently under consideration for patients with no established peritoneal metastases at the time of primary surgery; but with a clearly elevated risk of doing so postoperatively. Such acknowledged risk factors for developing peritoneal metastases include: T4 status of the primary tumor, perforated primary tumor, mucinous tumor, isolated ovarian metastases, positive lavage cytology, signet cell morphology and obstructed tumors (58). These patients may benefit from applying laparoscopic HIPEC at the time of the initial laparoscopic surgery.
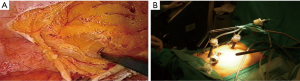
Ferron et al. were the first to explore the technical feasibility of laparoscopic CRS and HIPEC in a pig model (59). In a follow-up pharmacokinetic study they demonstrated an increased tissue diffusion of oxaliplatin during laparoscopic HIPEC when compared to open HIPEC (60). In 2006 our team presented the first human application of laparoscopic CRS and HIPEC (Figure 4) in a patient with limited peritoneal disease from appendiceal origin (61). Several groups have since confirmed feasibility and favorable short outcome in small retrospective series (62-67). Table 6 summarizes the current experience in combined laparoscopic CRS and HIPEC. Only very selected patients with limited disease and favorable histology are candidates for this approach.
Full table
A more viable application of laparoscopic HIPEC concerns the patients without synchronous peritoneal metastases but at high risk of developing them in the postoperative months after the resection of the primary tumor. Elias et al. reported undetected peritoneal metastases in 23 of the 41 (56%) patients who received mandatory second look laparotomy at one year after curative intent resection of a high risk colon cancer (tumor perforation, isolated ovarian metastases or minimal peritoneal disease) (68). Based on this rationale; Sammartino et al. demonstrated a survival benefit in a non-randomized trial using this proactive use of HIPEC in patients at high risk of developing peritoneal metastases at the time of initial surgery (69). In the next step, several randomized trials (PROPHYLOCHIP, GASTRICHIP, PROMENADE) explore the strategy of surgery plus HIPEC in high risk patients (30,70,71).
HIPEC moving up in the timeline of peritoneal surface malignancy implies that both their primary disease and the minimal peritoneal component (actual or high risk) become candidates for a laparoscopic approach. In 2010, Lygidakis et al. reported on laparoscopic HIPEC as an adjuvant modality within 20 days after initial laparoscopic rectal resection (n=87) for high-risk rectal cancer (72). Among forty patients who completed the two year follow-up, two patients developed local recurrence. A similar risk-reducing laparoscopic approach was taken by Fish et al. in 10 patients with low-grade appendiceal mucinous neoplasm (73). At 11 months follow up no evidence of disease progression was found. After demonstrating the safety and feasibility of adjuvant laparoscopic HIPEC in high risk colorectal cancer patients (74), the Dutch Colorectal Cancer Group has embarked on a randomized multicenter trial (COLOPEC) where both laparoscopic and open adjuvant HIPEC are investigated in the same patient group (75).
Laparoscopy in the palliation of malignant ascites
Malignant ascites is defined as an abnormal accumulation of fluid in the peritoneal cavity of cancer patients with intraperitoneal dissemination of their disease. Decreased lymphatic absorption due to tumor implants and increased fluid production due to altered capillary permeability are contributing features of the ascites formation (76-81). The most common clinical feature is a progressive increase of abdominal distension resulting in pain, discomfort, anorexia and dyspnea. This condition severely impairs the quality of life of these cancer patients in their terminal stages of disease (82,83). Iterative paracentesis, diuretics and albumin perfusion are used to treat cirrhotic ascites and might be useful to treat malignant ascites at its beginning, but lose effectiveness over time because of different underlying physiology (84). Other therapeutic approaches include radio-labeled antibodies, peritoneo-venous shunts and biologic agents as anti-VEGF molecules, metalloproteinase inhibitors and immuno-modulators (85-92). None of these approaches have been established as standard of care because of limited efficacy or severe side-effects. Palliative laparoscopic HIPEC has been explored to treat debilitating malignant ascites (93-97). Valle et al. in 52 patients with refractory malignant ascites observed one clinical recurrence of the ascites after laparoscopic HIPEC. An important improvement in performance status was observed postoperatively. The Karnofsky index increased with an average 20 points in the postoperative period. Abdominal sclerosis and induction of dense adhesions rather than direct cytotoxic effect of the IP drug are probably the major factor of efficacy of this technique. Ozols and co-authors in their phase I study reported sclerosing peritonitis and subsequent pain as the dose-limiting factor at 18 µM when performing intracavitary chemotherapy with doxorubicin in patients with advanced ovarian cancer (98).
In conclusion laparoscopy is a viable emerging tool in the diagnosis, staging and treatment of peritoneal surface malignancy patients, especially in the current era where CRS and HIPEC are moving up in the timeline of treatment algorithms for the patients. The limited amount of data warrants a cautious approach as to the application. It should be confined to centers with a proven track record in both laparoscopy and treating peritoneal surface malignancies. All patients should be part of an investigational study to gather more relevant data.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sugarbaker PH. Pseudomyxoma peritonei and peritoneal metastases from appendiceal malignancy. In: Sugarbaker PH, editor. Cytoreductive Surgery & Perioperative Chemotherapy for Peritoneal Surface Malignancy. Textbook and Video Atlas. Woodbury: Cine-Med Publishing, 2012:57-78.
- Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer 2010;116:5608-18. [PubMed]
- Bakrin N, Bereder JM, Decullier E, et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol 2013;39:1435-43. [PubMed]
- Spiliotis J, Halkia E, Lianos E, et al. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol 2015;22:1570-5. [PubMed]
- Glehen O, Mithieux F, Osinsky D, et al. Surgery combined with peritonectomy procedures and intraperitoneal chemohyperthermia in abdominal cancers with peritoneal carcinomatosis: a phase II study. J Clin Oncol 2003;21:799-806. [PubMed]
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737-43. [PubMed]
- Esquivel J, Piso P, Verwaal V, et al. American Society of peritoneal surface malignancies opinion statement on defining expectations from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with colorectal cancer. J Surg Oncol 2014;110:777-8. [PubMed]
- Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol 2006;7:69-76. [PubMed]
- Mohamed F, Cecil T, Moran B, et al. A new standard of care for the management of peritoneal surface malignancy. Curr Oncol 2011;18:e84-96. [PubMed]
- Nissan A, Stojadinovic A, Garofalo A, et al. Evidence-based medicine in the treatment of peritoneal carcinomatosis: Past, present, and future. J Surg Oncol 2009;100:335-44. [PubMed]
- Elias D, Goéré D, Dumont F, et al. Role of hyperthermic intraoperative peritoneal chemotherapy in the management of peritoneal metastases. Eur J Cancer 2014;50:332-40. [PubMed]
- Brücher BL, Piso P, Verwaal V, et al. Peritoneal carcinomatosis: cytoreductive surgery and HIPEC--overview and basics. Cancer Invest 2012;30:209-24. [PubMed]
- Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 2010;28:63-8. [PubMed]
- da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg 2006;203:878-86. [PubMed]
- Sugarbaker PH, Welch L, Mohamed F, et al. A review of peritoneal mesothelioma at the Washington Cancer Institute. Surg Oncol Clin N Am 2003;12:605-21. [PubMed]
- Landrum LM, Java J, Mathews CA, et al. Prognostic factors for stage III epithelial ovarian cancer treated with intraperitoneal chemotherapy: a Gynecologic Oncology Group study. Gynecol Oncol 2013;130:12-8. [PubMed]
- Van der Speeten K, Stuart OA, Sugarbaker PH. Pharmacology of perioperative intraperitoneal and intravenous chemotherapy in patients with peritoneal surface malignancy. Surg Oncol Clin N Am 2012;21:577-97. [PubMed]
- Sugarbaker PH, Graves T, DeBruijn EA, et al. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: pharmacologic studies. Cancer Res 1990;50:5790-4. [PubMed]
- Hompes D, D’Hoore A, Wolthuis A, et al. The use of Oxaliplatin or Mitomycin C in HIPEC treatment for peritoneal carcinomatosis from colorectal cancer: a comparative study. J Surg Oncol 2014;109:527-32. [PubMed]
- Prada-Villaverde A, Esquivel J, Lowy AM, et al. The American Society of Peritoneal Surface Malignancies evaluation of HIPEC with Mitomycin C versus Oxaliplatin in 539 patients with colon cancer undergoing a complete cytoreductive surgery. J Surg Oncol 2014;110:779-85. [PubMed]
- Sugarbaker PH, Mora JT, Carmignani P, et al. Update on chemotherapeutic agents utilized for perioperative intraperitoneal chemotherapy. Oncologist 2005;10:112-22. [PubMed]
- Van der Speeten K, Stuart OA, Mahteme H, et al. Pharmacokinetic Study of Perioperative Intravenous Ifosfamide. Int J Surg Oncol 2011;2011:185092.
- Elias D, Bonnay M, Puizillou JM, et al. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: Pharmacokinetics and tissue distribution. Ann Oncol 2002;13:267-72. [PubMed]
- Sugarbaker PH, Kern K, Lack E. Malignant pseudomyxoma of colonic origin. Natural history and presentation of a curative approach to treatment. Dis Colon Rectum 1987;30:772-9. [PubMed]
- Sethna KS, Sugarbaker PH. New prospects for the control of peritoneal surface dissemination of gastric cancer using perioperative intraperitoneal chemotherapy. Cancer Therapy 2004;2:79-84.
- Warshaw AL, Fernández-del Castillo C. Pancreatic carcinoma. N Engl J Med 1992;326:455-65. [PubMed]
- Stojadinovic A, Pisters PW, Kelsen DP, et al. Cancer of the stomach. In: De Vita VT, Lawrence T, Rosenberg S, editors. Cancer: Principles and Practice of Oncology, 10th Edition. Philadelphia: Lippincott Williams & Wilkins 2015:613-50.
- Sugarbaker PH. Second-look surgery for colorectal cancer: revised selection factors and new treatment options for greater success. Int J Surg Oncol 2011;2011:915078.
- Adjuvant HIPEC in High Risk Colon Cancer (COLOPEC). Available online: https://clinicaltrials.gov/ct2/show/NCT02231086
- Sammartino P, Societa Italiana di Chirurgia Oncologica (SICO). PROMENADE Trial (PROactive Management of ENdoperitoneal spreAD in colonic cancer).2015.
- Urano M, Kuroda M, Nishimura Y. Invited review for the clinical application of thermochemotherapy given at mild temperatures. Int J Hyperthermia 1999;15:79-107. [PubMed]
- Elias D, Raynard B, Bonnay M, et al. Heated intra-operative intraperitoneal oxaliplatin alone and in combination with intraperitoneal irinotecan: Pharmacologic studies. Eur J Surg Oncol 2006;32:607-13. [PubMed]
- Van der Speeten K, Stuart OA, Chang D, et al. Changes induced by surgical and clinical factors in the pharmacology of intraperitoneal mitomycin C in 145 patients with peritoneal carcinomatosis. Cancer Chemother Pharmacol 2011;68:147-56. [PubMed]
- Sugarbaker PH, Van der Speeten K, Stuart OA, et al. Impact of surgical and clinical factors on the pharmacology of intraperitoneal doxorubicin in 145 patients with peritoneal carcinomatosis. Eur J Surg Oncol 2011;37:719-26. [PubMed]
- Sticca RP, Dach BW. Rationale for hyperthermia with intraoperative intraperitoneal chemotherapy agents. Surg Oncol Clin N Am 2003;12:689-701. [PubMed]
- Sugarbaker PH. Laboratory and clinical basis for hyperthermia as a component of intracavitary chemotherapy. Int J Hyperthermia 2007;23:431-42. [PubMed]
- Jacquet P, Averbach A, Stuart OA, et al. Hyperthermic intraperitoneal doxorubicin: Pharmacokinetics, metabolism, and tissue distribution in a rat model. Cancer Chemother Pharmacol 1998;41:147-54. [PubMed]
- Young JS, Lumsden CE, Stalker AL. The significance of the tissue pressure of normal testicular and of neoplastic (Brown-Pearce carcinoma) tissue in the rabbit. J Pathol Bacteriol 1950;62:313-33. [PubMed]
- Leunig M, Goetz AE, Dellian M, et al. Interstitial fluid pressure in solid tumors following hyperthermia: possible correlation with therapeutic response. Cancer Res 1992;52:487-90. [PubMed]
- Sugarbaker PH, Stuart OA, Bijelic L, et al. Hyperthermic intraperitoneal gemcitabine chemotherapy for patients with resected pancreatic cancer: Clinical pharmacologic data. Current Topics in Pharmacology 2014;18:81-92.
- Harrison LE, Tiesi G, Razavi R, et al. A phase I trial of thermal sensitization using induced oxidative stress in the context of HIPEC. Ann Surg Oncol 2013;20:1843-50. [PubMed]
- Lang-Lazdunski L, Bille A, Papa S, et al. Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine, prophylactic radiotherapy, and systemic chemotherapy in patients with malignant pleural mesothelioma: a 10-year experience. J Thorac Cardiovasc Surg 2015;149:558-65; discussion 565-6. [PubMed]
- Kuramoto M, Shimada S, Ikeshima S, et al. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg 2009;250:242-6. [PubMed]
- Sugarbaker PH. An overview of peritonectomy, visceral resections, and perioperative chemotherapy for peritoneal surface malignancy. In: Sugarbaker PH, editor. Cytoreductive Surgery & Perioperative Chemotherapy for Peritoneal Surface Malignancy. Textbook and Video Atlas. Woodbury: Cine-Med Publishing, 2012:1-30.
- Sugarbaker PH, Averbach AM, Jacquet P, et al. A simplified approach to hyperthermic intraoperative intraperitoneal chemotherapy (HIIC) using a self retaining retractor. In: Sugarbaker PH, editor. Peritoneal Carcinomatosis: Principles of Management. Boston: Springer US, 1996.
- Benoit L, Cheynel N, Ortega-Deballon P, et al. Closed hyperthermic intraperitoneal chemotherapy with open abdomen: a novel technique to reduce exposure of the surgical team to chemotherapy drugs. Ann Surg Oncol 2008;15:542-6. [PubMed]
- Pasqual EM, Bertozzi S, Bacchetti S, et al. Preoperative assessment of peritoneal carcinomatosis in patients undergoing hyperthermic intraperitoneal chemotherapy following cytoreductive surgery. Anticancer Res 2014;34:2363-8. [PubMed]
- Laterza B, Kusamura S, Baratti D, et al. Role of explorative laparoscopy to evaluate optimal candidates for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal mesothelioma. In Vivo 2009;23:187-90. [PubMed]
- Garofalo A, Valle M. Laparoscopy in the management of peritoneal carcinomatosis. Cancer J 2009;15:190-5. [PubMed]
- Pomel C, Appleyard TL, Gouy S, et al. The role of laparoscopy to evaluate candidates for complete cytoreduction of peritoneal carcinomatosis and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol 2005;31:540-3. [PubMed]
- Fagotti A, Vizzielli G, De Iaco P, et al. A multicentric trial (Olympia-MITO 13) on the accuracy of laparoscopy to assess peritoneal spread in ovarian cancer. Am J Obstet Gynecol 2013;209:462.e1-462.e11.
- Valle M, Federici O, Garofalo A. Patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy, and role of laparoscopy in diagnosis, staging and treatment. Surg Oncol Clin N Am 2012;21:515-31. [PubMed]
- Thomassen I, Van Gestel YR, Aalbers AG, et al. Peritoneal carcinomatosis is less frequently diagnosed during laparoscopic surgert compared to open surgery in patients with colorectal cancer. Eur J Surg Oncol 2014;40:511-4. [PubMed]
- Varnoux C, Huchon C, Bats AS, et al. Diagnostic accuracy of hand-assisted laparoscopy in predicting resectability of peritoneal carcinomatosis from gynecological malignancies. Eur J Surg Oncol 2013;39:774-9. [PubMed]
- von Breitenbuch P, Jeiter T, Schreml S, et al. Autofluorescent imaging in patients with peritoneal carcinomatosis. Surg Innov 2014;21:187-93. [PubMed]
- Vennix S, Pelzers L, Bouvy N, et al. Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev 2014;4:CD005200. [PubMed]
- Wang CL, Qu G, Xu HW. The short- and long-term outcomes of laparoscopic versus open surgery for colorectal cancer: a meta-analysis. Int J Colorectal Dis 2014;29:309-20. [PubMed]
- Honoré C, Goéré D, Souadka A, et al. Definition of patients presenting a high risk of developing peritoneal carcinomatosis after curative surgery for colorectal cancer: a systematic review. Ann Surg Oncol 2013;20:183-92. [PubMed]
- Ferron G, Gesson-Paute A, Classe JM, et al. Feasibility of laparoscopic peritonectomy followed by intra-peritoneal chemohyperthermia: an experimental study. Gynecol Oncol 2005;99:358-361. [PubMed]
- Thomas F, Ferron G, Gesson-Paute A, et al. Increased tissue diffusion of oxaliplatin during laparoscopically assisted versus open heated intraoperative intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol 2008;15:3623-4. [PubMed]
- Van der Speeten K. Laparoscopic HIPEC: initial experience, opportunities and limitations. Poster presentation. 5th International Workshop on Peritoneal Surface Malignancy. Milano, Italy, 2006.
- Esquivel J, Averbach A. Laparoscopic Cytoreductive Surgery and HIPEC in Patients with Limited Pseudomyxoma Peritonei of Appendiceal Origin. Gastroenterol Res Pract 2012;2012:981245.
- Knutsen A, Sielaff TD, Greeno E, et al. Staged laparoscopic infusion of hyperthermic intraperitoneal chemotherapy after cytoreductive surgery. J Gastrointest Surg 2006;10:1038-43. [PubMed]
- Hirano M, Yonemura Y, Canbay E, et al. Laparoscopic Diagnosis and Laparoscopic Hyperthermic Intraoperative Intraperitoneal Chemotherapy for Pseudomyxoma Peritonei Detected by CT Examination. Gastroenterol Res Pract 2012;2012:741202.
- Esquivel J, Averbach A. Combined laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a patient with peritoneal mesothelioma. J Laparoendosc Adv Surg Tech A 2009;19:505-7. [PubMed]
- Passot G, Bakrin N, Isaac S, et al. Postoperative outcomes of laparoscopic vs open cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for treatment of peritoneal surface malignancies. Eur J Surg Oncol 2014;40:957-62. [PubMed]
- Fagotti A, Petrillo M, Costantini B, et al. Minimally invasive secondary cytoreduction plus HIPEC for recurrent ovarian cancer: a case series. Gynecol Oncol 2014;132:303-6. [PubMed]
- Elias D, Honoré C, Dumont F, et al. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg 2011;254:289-93. [PubMed]
- Sammartino P, Sibio S, Biacchi D, et al. Long-term results after proactive management for locoregional control in patients with colonic cancer at high risk of peritoneal metastases. Int J Colorectal Dis 2014;29:1081-9. [PubMed]
- Elias D, Dekkal M. Multicentric Phase III Trial Comparing Simple Follow-up to Exploratory Laparotomy Plus "in Principle" HIPEC (Hyperthermic Intraperitoneal Chemotherapy) in Colorectal Patients Initially Treated With Surgery and Adjuvant Chemotherapy Who Have a High Risk of Developing Colorectal Peritoneal Carcinomatosis.
- Glehen O, Passot G, Villeneuve L, et al. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC Cancer 2014;14:183. [PubMed]
- Lygidakis NJ, Patil A, Giannoulis K, et al. Laparoscopic hyperthermic intraperitoneal chemotherapy as adjuvant modality following radical surgery for advanced rectal cancer a new look to an old problem. Hepatogastroenterology 2010;57:73-5. [PubMed]
- Fish R, Selvasekar C, Crichton P, et al. Risk-reducing laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for low-grade appendiceal mucinous neoplasm: early outcomes and technique. Surg Endosc 2014;28:341-5. [PubMed]
- Sloothaak DA, Gardenbroek TJ, Crezee J, et al. Feasibility of adjuvant laparoscopic hyperthermic intraperitoneal chemotherapy in a short stay setting in patients with colorectal cancer at high risk of peritoneal carcinomatosis. Eur J Surg Oncol 2014;40:1453-8. [PubMed]
- Klaver CE, Musters GD, Bemelman WA, et al. Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with colon cancer at high risk of peritoneal carcinomatosis; the COLOPEC randomized multicentre trial. BMC Cancer 2015;15:428. [PubMed]
- Tamsma J. The pathogenesis of malignant ascites. Cancer Treat Res 2007;134:109-18. [PubMed]
- Adam RA, Adam YG. Malignant ascites: past, present, and future. J Am Coll Surg 2004;198:999-1011. [PubMed]
- Sako A, Kitayama J, Shida D, et al. Lysophosphatidic acid (LPA)-induced vascular endothelial growth factor (VEGF) by mesothelial cells and quantification of host-derived VEGF in malignant ascites. J Surg Res 2006;130:94-101. [PubMed]
- Nagy JA, Morgan ES, Herzberg KT, et al. Pathogenesis of ascites tumor growth: angiogenesis, vascular remodeling, and stroma formation in the peritoneal lining. Cancer Res 1995;55:376-85. [PubMed]
- Coates G, Bush RS, Aspin N. A study of ascites using lymphoscintigraphy with 99 m Tc-sulfur colloid. Radiology 1973;107:577-83. [PubMed]
- Garrison RN, Kaelin LD, Galloway RH, et al. Malignant ascites. Clinical and experimental observations. Ann Surg 1986;203:644-51. [PubMed]
- Easson AM, Bezjak A, Ross S, et al. The ability of existing questionnaires to measure symptom change after paracentesis for symptomatic ascites. Ann Surg Oncol 2007;14:2348-57. [PubMed]
- Becker G, Galandi D, Blum HE. Malignant ascites: systematic review and guideline for treatment. Eur J Cancer 2006;42:589-97. [PubMed]
- Cavazzoni E, Bugiantella W, Graziosi L, et al. Malignant ascites: pathophysiology and treatment. Int J Clin Oncol 2013;18:1-9. [PubMed]
- Lee CW, Bociek G, Faught W. A survey of practice in management of malignant ascites. J Pain Symptom Manage 1998;16:96-101. [PubMed]
- Ariel IM, Oropeza R, Pack GT. Intracavitary administration of radioactive isotopes in the control of effusions due to cancer. Results in 267 patients. Cancer 1966;19:1096-102. [PubMed]
- Leveen HH, Christoudias G, Ip M, et al. Peritoneo-venous shunts for ascites. Ann Surg 1974;180:580-91. [PubMed]
- Oosterlee J. Peritoneovenous shunts for ascites in cancer patients. Br J Surg 1980;67:663-6. [PubMed]
- Xu L, Yoneda J, Herrera C, et al. Inhibition of malignant ascites and growth of human ovarian carcinoma by oral administration of a potent inhibitor of the vascular endothelial growth factor receptor tyrosine kinases. Int J Oncol 2000;16:445-54. [PubMed]
- Parsons SL, Watson SA, Steele RJ. Phase I/II trial of batimastat, a matrix metalloproteinase inhibitor, in patients with malignant ascites. Eur J Surg Oncol 1997;23:526-31. [PubMed]
- Katano M, Morisaki T. The past, the present and future of the OK-432 therapy for patients with malignant effusions. Anticancer Res 1998;18:3917-25. [PubMed]
- Frampton JE. Catumaxomab: in malignant ascites. Drugs 2012;72:1399-410. [PubMed]
- Garofalo A, Valle M, Garcia J, et al. Laparoscopic intraperitoneal hyperthermic chemotherapy for palliation of debilitating malignant ascites. Eur J Surg Oncol 2006;32:682-5. [PubMed]
- Facchiano E, Scaringi S, Kianmanesh R, et al. Laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) for the treatment of malignant ascites secondary to unresectable peritoneal carcinomatosis from advanced gastric cancer. Eur J Surg Oncol 2008;34:154-8. [PubMed]
- Patriti A, Cavazzoni E, Graziosi L, et al. Successful palliation of malignant ascites from peritoneal mesothelioma by laparoscopic intraperitoneal hyperthermic chemotherapy. Surg Laparosc Endosc Percutan Tech 2008;18:426-8. [PubMed]
- Valle M, Van der Speeten K, Garofalo A. Laparoscopic hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) in the management of refractory malignant ascites: A multi-institutional retrospective analysis in 52 patients. J Surg Oncol 2009;100:331-4. [PubMed]
- de Mestier L, Volet J, Scaglia E, et al. Is palliative laparoscopic hyperthermic intraperitoneal chemotherapy effective in patients with malignant hemorrhagic ascites? Case Rep Gastroenterol 2012;6:166-70. [PubMed]
- Ozols RF, Young RC, Speyer JL, et al. Phase I study and pharmacological studies of adriamycin administered intraperitoneally to patients with ovarian cancer. Cancer Res 1982;42:4265-9. [PubMed]


