Then and now: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective
Introduction
Peritoneal carcinomatosis remains a devastating manifestation of advanced intra-abdominal malignancies. Management and therapy of this clinical entity, which balance efforts to extend survival and improve quality of life, have been challenging and have evolved significantly over the last century (Figure 1). Substantial strides have been made in the surgical arena to achieve safe clearance of macroscopic disease by means of cytoreductive surgery (CRS) and more recently, combined with hyperthermic intraperitoneal chemotherapy (HIPEC), for treatment of microscopic or minimal volume disease.
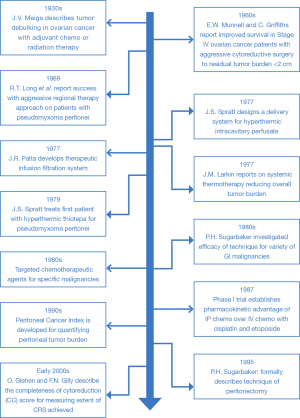
Extensive operative debulking procedures were initially described as early as the 1930s for locally advanced ovarian cancers. Over the subsequent decades, the utilization of intraperitoneal chemotherapy was explored and applied to peritoneal metastases of ovarian origin and eventually to other GI malignancies. In the 1970s and 1980s, hyperthermia was also increasingly employed with intraperitoneal chemotherapy to increase the efficacy and potency of the anti-neoplastic agents. This procedure of HIPEC in conjunction with CRS has since emerged as an important means of controlling peritoneal disease and has been associated with favorable survival outcomes in selected patients. It is probably fair to say there has been a paradigm shift in how certain malignancies with peritoneal dissemination are viewed from representing a “systemic” problem unamenable to surgical therapy, to a “locoregional” problem for which aggressive regional approaches may be warranted. This has been driven in part by an improved understanding of the tumor biology of certain malignancies and the effective scope of systemic therapies. Over time, as new drugs and technologies are developed and further steps are made in the understanding of tumor biology, the application of HIPEC will likely continue to evolve. This article briefly outlines the history of CRS and HIPEC, highlighting some of the significant events in its development from inception to the present day.
Early developments in CRS
The important role of primary CRS was established over 80 years ago in locally advanced abdominal malignancies. In 1934, Dr. Meigs in New York originally described tumor debulking surgery for ovarian cancer under the premise that reducing macroscopic disease burden would ameliorate patient symptoms and reduce complications such as intestinal obstruction, perforation, ascites, etc. (2). Post-operative radiotherapy, and occasionally chemotherapy were also used in the adjuvant setting to further improve local control (3,4).
Aggressive cytoreductive approaches did not take hold as a mainstay of operative management in ovarian carcinomatosis until the late 1960s and early 1970s. Single-institution experiences in patients with stage I-III ovarian cancers demonstrated an association between extensive tumor debulking at the time of surgery and improved survival outcomes (5,6). During this time, Dr. Griffths at the National Cancer Institute also reported on prognostic indicators of survival in stage II and III ovarian cancer patients, importantly noting that residual tumor mass size (<1.6 cm) after CRS was significantly associated with extended survival (7). Survival rates also appeared to be improved with the addition of post-operative intravenous (IV) chemotherapy (8). There were several rationales for use of CRS for improving the efficacy of drug therapy in the “adjuvant” setting. These included allowing for better drug delivery to smaller tumor implants with adequate perfusion as well as the concept that smaller masses in early growth phases would be more chemosensitive. Removal of larger, necrotic tumor implants in areas of potential bowel perforation and obstruction could also aid in improving the nutritional status of patients.
Management of pseudomyxoma peritonei (PMP), arising from appendiceal and less commonly ovarian mucinous tumors, followed suit with early debulking operations, in attempts to reduce the effects of the large, space occupying mucoid collections. As distant metastatic disease is relatively rare in these cancers, complications and morbidity are typically the sequelae of obstruction and erosion of tumor masses and mucinous material. Moreover, systemic therapy is frequently ineffective for reducing tumor bulk in these patients. Aggressive CRS therefore developed as the mainstay of management and palliation. In 1969, a group out of Alabama, Long et al. reported long term survival outcomes in select groups of patients with PMP undergoing graduated levels of surgical intervention with adjuvant chemotherapy or radiotherapy. Although overall cohort numbers were small, they found that those patients undergoing multiple cytoreductive operations along with administration of alkylating agents (intraperitoneal or oral) had markedly increased survival rates compared with less aggressive methods (9). Similar findings of improved survival with aggressive removal of large mucinous implants and excision of involved peritoneum were reported in a large experience by the Memorial Sloane-Kettering Cancer Center from 1950-1970 (10).
Role for intraperitoneal chemotherapy with hyperthermia
The 1970s saw increasing utilization of adjuvant therapy, including radiation, IV chemotherapy and intraperitoneal chemotherapy. Insight was increasingly gained into the pharmokinetic differences between IV and intraperitoneal chemotherapy administration for treatment of peritoneal tumors (11). A trial comparing IV and IP routes of cisplatin administration for toxicity levels in canines showed similar IV levels after 4 hours but significantly higher levels in the peritoneal cavity after IP administration (12). This supported the concept that IP chemotherapy could improve the therapeutic index for treatment of peritoneal disease. Around this time, Dr. Palta at the University of Missouri-Columbia was in the process of developing a filtration system for intraperitoneal infusion of chemotherapeutic agents (13). Meanwhile the impact of systemic and total body hyperthermia on patients with advanced cancers was being investigated for decreasing overall tumor burden (14). Additional studies of isolated perfusion of visceral vasculature with hyperthermic chemotherapy were being performed with early positive results (15).
Spratt et al. at the University of Louisville in Kentucky combined these concepts into a thermal transfusion infiltration system (TIFS) for delivery of heated chemotherapy into the peritoneal space of canines (16). This involved the recirculation of intra-cavitary effusions, filtration of debris, maintenance of drug concentration and homogenous temperature distribution. Ultimately, their system allowed for infusion of temperature-controlled hyperthermic agents at large volumes without significantly raising core body temperature or causing other significant complications. They soon applied their TIFS perfusion procedure to a 35-year-old male suffering from recurrent PMP. The patient had previously undergone a laparotomy with cytoreduction, and had been actively pursuing additional treatment. At his insistence, in 1979, the patient became the first human subjected to TIFS with administration of hyperthermic chemotherapy for locally advanced abdominal malignancy (17). He underwent removal of his gross disease recurrence followed by TIFS to deliver heated intraperitoneal Thiotepa. This was followed by infusion of intraperitoneal methotrexate on post-operative day 5 through catheters, which had been left in place, and the patient survived the operation and was discharged without significant complications.
Further studies continued into the early 1980s with peritoneal carcinomatosis and PMP primarily from ovarian and appendiceal malignancies. The role for direct drug delivery to the peritoneal and tumor surfaces was described and reported in multiple reviews of cisplatin administration (18,19). Chemotherapeutic agents were delivered intraperitoneally at concentrations up to 30 times greater than those safely administered via IV route. Furthermore, intraperitoneal clearance of the agents also allowed for some amount of drug delivery by absorption and subsequent IV perfusion, which allowed for a ‘double-dosing’ of chemotherapy and overall reduction in systemic toxicities.
Initial trials with CRS and HIPEC
Phase I trials were underway by the mid 1980s to demonstrate the quantitative pharmacokinetic advantages of intra-peritoneal chemotherapy over systemic chemotherapy. One of the first phase I trials to achieve this goal documented the antineoplastic activity of cisplatin and etoposide in 1987 (20). This group later reported on long-term survival in patients with ovarian carcinomatosis receiving IP therapy, with mean survival greater than 49 months in patients with residual tumors less than 2 cm (21). Multiple phase II trials were also completed during this decade, providing further clinical experience and early outcomes, primarily involving patients with ovarian cancer (22). Further investigation into therapy for gastrointestinal malignancies with peritoneal dissemination was spearheaded by Dr. Sugarbaker at the Washington Cancer Institute with early reports of survival benefits (23). Additional prospective studies were performed looking at prognostic indicators of survival and the impact of neoadjuvant chemotherapy in patients undergoing HIPEC for gastrointestinal malignancies (24,25).
With early promising data for peritoneal carcinomatosis of multiple origins, interest quickly spread throughout the surgical and scientific communities in hope of achieving more optimal palliation and ultimately more favorable outcomes for these patients. In Japan, preclinical studies investigating continuous HIPEC in rats with implanted hepatoma carcinomatosis demonstrated prolonged survival in groups receiving hyperthermic mitomycin C perfusion compared with controls or with groups receiving hyperthermia or mitomycin C alone (26). Similar early clinical trials were carried out in small cohorts of patients with disseminated gastric cancers (27,28). While these studies reported relatively short-term follow-up, complication rates were low and patients demonstrated encouraging survival rates. Further studies began investigating other chemotherapeutic agents for intraperitoneal infusion in managing tumors of differing histologies. Cisplatin was evaluated among patients with colorectal and gastric cancers, while 5-fluorouracil was tested in patients with colorectal primaries (29,30). Meanwhile, platinum-based therapy remained at the forefront in IPC trials for ovarian carcinomatosis during the 1980s (18,19,22).
Optimizing CRS
As strides in the intraperitoneal delivery of hyperthermic chemotherapy were being made, the important need for standardization of CRS was recognized. It became apparent fairly early on that completeness of cytoreduction (CC) was associated with survival outcomes. In ovarian cancer, one early study demonstrated response to therapy and survival benefits were most pronounced when largest intraperitoneal tumor implants were <2 cm (21). Multiple studies with gastrointestinal malignancies found similar results correlating residual tumor volume with outcomes. Development of methods for assessing complete resectability of tumors (pre-operatively and intra-operatively) and safe and systematic techniques by which to achieve these goals were important developments in CRS. Moreover, extensive removal of involved peritoneum during resection was often an important component of the CRS, particularly for primary tumors of the peritoneum (i.e., mesothelioma). A stepwise approach to this component of CRS was outlined by Dr. Sugarbaker in 1995 (31). The methods described were meant to be applicable to peritoneal carcinomatosis of various etiologies. Six separate peritonectomy procedures were described: omentectomy with splenectomy, left upper quadrant peritonectomy, right upper quadrant peritonectomy, lesser omentectomy with cholecystectomy, antrectomy and stripping of omental bursa, and pelvic peritonectomy including colorectal resection. These techniques were utilized in conjunction with IPC as appropriate in subsequent studies (32,33). Sugarbaker outlined detailed surgical techniques describing peritonectomy and associated organ resection in upper quadrants and in the pelvis, as well as omental and bowel resections as indicated for tumor involvement.
Intraperitoneal delivery techniques
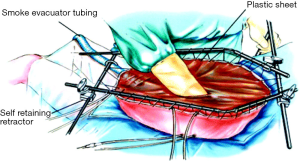
Multiple modalities of delivery of HIPEC therapy have been described and employed over time with advantages and disadvantages recognized for each (34). The open approach or “Coliseum” technique, involves a silicon plastic sheet placed over a retractor apparatus and the open abdominal cavity and is secured to the skin. An incision is made through the slit to expose the abdominal contents and form an elevated cavity or coliseum to allow for perfusion of the hyperthermic chemotherapy solution.
A similar approach described by Dr. Sugarbaker in 1999 involves instillation of the chemotherapy with a Tenckhoff catheter (Figure 2) (36). Benefits of this open approach included direct access by the surgeon to the cavity during administration of the hyperthermic agents to manipulate the fluid and bowel in order to achieve a quick and homogenous temperature and distribution of drug within the abdomen. Additionally, care can be taken to ensure that all peritoneal surfaces are exposed equally throughout the duration of the therapy as well as avoid dangerous temperatures or over-exposure to normal tissues. Potential downsides to the procedure are rapid dissipation of heat, requiring more involved efforts to maintain ideal hyperthermic temperatures, and the potential exposure of surgeons and operating staff to chemotherapy agents both by direct contact and aerosolized particles (34).
In comparison, the closed technique involves the closure of the abdominal wall prior to infusion of the chemotherapy reducing the issue of heat loss from peritoneal surfaces (Figure 3). Furthermore it provides a space in which flow rates can be maintained for homogenous hyperthermia and exposure as well as instillation of positive pressure to enhance drug penetration (38). The major disadvantage of the closed technique is the uneven distribution of chemotherapeutic agents within the peritoneal cavity, leading to pooling of fluids and accumulation of toxic concentrations of agents and heat (39). This can be alleviated to some degree with manual external agitation.
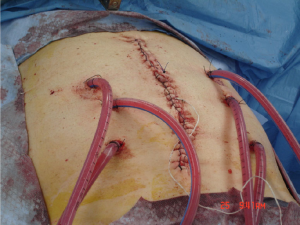
In attempts to combine potential advantages of these two techniques, alternative methods using a semi-open approach or an apparatus known as a peritoneal cavity expander (PCE) were developed. The semi-open technique involves the use of an abdominal cavity expander supported by a retractor and secured to the skin edges in a watertight fashion with a stapler device. The edge of the apparatus can then be elevated above skin level to allow for extensive manipulation of abdominal contents while maintaining homogenous hyperthermia without loss of fluids (40). Dr. Sugarbaker employed this semi-open method by developing a new containment instrument (Thompson retractor) described in 2005 to support watertight elevation of the abdominal skin edges (41). This again creates a reservoir for the chemohyperthermic fluids and an access site for manual agitation of intra-abdominal contents.
The PCE also minimizes loss of heat while maintaining homogeneity. Formally described by Fujimura et al. in 1990, the PCE is an acrylic, cylinder shaped container, which is suspended on each end with a flange (42). One flange is affixed to the abdominal opening while the other is suspended by two right-angled bars, which are anchored to the operating table. In addition to direct manipulation of the intraperitoneal cavity, the cylinder container allows for exteriorization of the small intestine to further ensure homogeneity in exposure to heat and chemotherapy. Both of these techniques decreased the amount of heat lost in a completely open method for HIPEC delivery; however they still pose the risk of exposure to agents by operating personnel. Additionally the PCE presents a relatively complex setup and requires significant operator expertise.
In the early 2000s, Elias et al. conducted a prospective phase I-II study evaluating a total of seven different delivery methods for HIPEC in 32 patients. They found that the closed abdomen techniques did not accomplish ideal thermal homogeneity and the open methods with upward retraction of skin edges were more consistent in this parameter (39). Subsequently, Glehen et al. surveyed an expert group of surgical oncologists on the best operative technique for HIPEC delivery with the consensus being that there is insufficient evidence to support the superiority of one single method over another and that a prospective randomized trial would be necessary to study this question (34).
More recently, a laparoscopic approach for CRS with HIPEC in highly selected patients with minimal disease burden has been described (43). This was successfully employed in a prospective series of 19 patients with limited carcinomatosis, with 1 conversion to open from laparoscopic. The mean length of hospital stay was relatively short (5.3 days), while short follow-up oncologic outcomes in these low tumor volume patients (100% survival with mean follow-up of 17 months) appeared favorable. Further studies are needed to better define the indications and outcomes of this approach.
Quantifying tumor burden and completeness of resection
Since most patients with peritoneal dissemination of disease would be classified as having stage IV disease, there was a need to develop a more specific language to quantify tumor burden both from the standpoint of prognosis and suitability for CRS. Multiple groups became interested in establishing patient selection criteria for CRS and HIPEC based on tumor implant size and extent of disease spread within the peritoneal cavity. Intuitively, increased tumor burden was seen as a negative prognostic factor for carcinomatosis treatment and survival. In a collective effort by Dr. Sugarbaker and colleagues, the Peritoneal Cancer Index (PCI) was developed in the 1990s and described in multiple publications (44-46). The score from this rating, ranging from 1-39, integrates lesion size and distribution of peritoneal involvement of disease. The abdomen is divided into 9 grid-like segments, numbered in a clockwise fashion [0-8]. The small intestine is further divided into 4 segments along its length (9-10 for jejunum and 11-12 for ileum) for a total of 13 intra-abdominal ‘regions’. The lesion size of the largest residual tumor found in each segment is then scored from LS-0 to LS-3. LS-0 designates no residual tumor seen, LS-1 for tumors up to 0.5 cm in greatest dimension, LS-2 for tumors 0.5-5.0 cm, and LS-3 for tumors >5.0 cm or a conglomerate of multiple deposits. Scoring is performed during intra-operative exploration and direct visualization of organ and peritoneal involvement. Each segment is assigned an individual score and the total is calculated to give the composite PCI.
The PCI score provided the opportunity to develop recommendations for suitability for debulking surgery based on tumor extent. Initially, CRS and HIPEC was recommended for patients with colorectal carcinomatosis with PCI score of less than 20, as this group exhibited 5-year survival rates with surgery of 20% (47); a subsequent study suggested more optimal PCI threshold for surgery in this group was 15 or lower (48). Outcomes and recommendations for surgery with PCI score have been designated based on tumor of origin. For instance, gastric carcinomatosis with PCI less than 15 (49) have more favorable outcomes compared with much higher PCI scores for PMP (50). The PCI “threshold” for surgery for a given tumor type therefore should take into account not only the likelihood of achieving complete cytoreduction, but also the specific tumor biology and effectiveness of other systemic (nonsurgical) therapies. Several similar staging schemata were developed by various organizations including the Gilly Peritoneal Carcinomatosis staging, the Japanese Staging system and the Dutch Simplified Peritoneal Cancer Index (51-53). While PCI at laparotomy was long considered the gold standard for predicting feasibility of successful cytoreduction to minimal residual tumor burden, less invasive modes of predicting extent of peritoneal involvement are also utilized including CT imaging and staging laparoscopy (54). It is important to note that identification of tumor involvement at various anatomic sites (i.e., biliary tree or small bowel serosa, diffuse lymphadenopathy) will understandably have negative impact on prognosis irrespective of PCI score.
The degree to which CRS is achieved has also been recognized as an important operative factor associated with prognosis. The CC score was developed in the early 2000s by Drs. Glehen and Gilly to theoretically predict likelihood of benefit from intraperitoneal therapy (54). Patients with no visible residual tumor after surgical debulking are given a score of CC-0, while those with largest residual tumor nodules <2.5 mm are given CC-1 scores. A cutoff of 2.5 mm was designated as the largest nodule size thought to be affected by intraperitoneal chemotherapy, rendering that patient free of macroscopic disease at the end of treatment. CC-2 is designated for largest tumor deposits between 2.5 mm and 2.5 cm in size and CC-3 is for tumors greater than 2.5 cm or confluence of multiple smaller nodules. Ideally, surgery with therapeutic intent is aimed at achieving CC of 1 or less (55). In a subgroup analysis of a randomized trial of patients with colorectal carcinomatosis undergoing CRS/HIPEC with systemic therapy versus systemic therapy alone, there appeared to be quickly diminishing advantages to the combined therapeutic approach with increasing CC score (56). In a multicentric retrospective study of patients with colorectal carcinomatosis Glehen et al. identified the CC score as the most significant independent prognostic factor associated with patient survival (57).
Clinical trials
With renewed interest in the pharmacokinetics of intra-peritoneal chemotherapy and the development of various delivery methods, further investigation in the form of multiple clinical trials burgeoned in the 1990s and early 2000s. The progressive group from Lyon, France designed the EVOCAPE I (Evolution of Peritoneal Carcinomatosis) study to evaluate peri-operative data and validate the methods of the CRS/HIPEC operation in non-gynecologic malignancies (58). This multicentric prospective trial provided extensive information on the natural history of peritoneal carcinomatosis as well effective pre-operative diagnostic and staging modalities, and early morbidity and mortality outcomes following CRS with HIPEC. The foundation was therefore provided for a follow-up trial, EVOCAPE II, to define patients with GI malignancies who are at high risk for development of peritoneal carcinomatosis throughout the course of their disease (59). Also a prospective, multi-centered study, the group followed patients with GI malignancies through surgery and post-operative follow-up for 2 years with primary endpoint of overall survival. They found that the TNM staging, tumor differentiation, performance status, ASA and location of primary tumor were significant independent prognostic factors while the presence of intraperitoneal free cancer cells was not significant.
Interest in directed HIPEC therapy for patients based on origin of primary tumor has continued to gain momentum. A randomized phase II trial was conducted by Fujimura et al. for gastric cancer in the early 1990s (60). They showed that peritoneal carcinomatosis recurrence rates were significantly reduced in normothermic and hyperthermic administration of intraperitoneal chemotherapy and studied thresholds of adequate concentration levels of intraperitoneal mitomycin C while maintaining systemically safe levels. Rossi et al. reported overall morbidity and mortality rates for patients undergoing HIPEC for gastric adenocarcinoma, concluding that CRS with HIPEC appeared a worthwhile procedure in improving disease free survival (61). A phase III randomized controlled trial was designed by a group in the Netherlands to determine efficacy of HIPEC with CRS in comparison to previously established standard of systemic chemotherapy without palliative surgery (56). Early results showed a significant benefit in disease free survival in the experimental HIPEC arm. These findings were confirmed in long-term follow-up, with 5-year survival rate of 45% reported for those patients who had complete CRS (62). In 2011, Yang et al. published results of a phase III randomized clinical trial evaluating the efficacy of HIPEC with CRS compared to CRS alone in patients with peritoneal carcinomatosis from gastric adenocarcinoma. Importantly, they demonstrated significantly prolonged survival in the group undergoing CRS with HIPEC (63). Another Phase III study in France (PRODIGE 7), is evaluating the specific contribution of HIPEC to CRS in patients with carcinomatosis of colorectal origin. A large multicenter Dutch trial (COLOPEC trial) is meanwhile evaluating the role of HIPEC in the adjuvant setting for patients with colon cancers with high risk of development of carcinomatosis (64).
Meanwhile, several large retrospective multicentric studies have evaluated outcomes in patients with colorectal cancers, mesothelioma and PMP undergoing CRS/HIPEC. In 2010, Elias et al. described independent prognostic indicators of survival in patients with peritoneal carcinomatosis from colorectal cancer undergoing HIPEC were completeness of CRS, decreased peritoneal disease and no lymph node involvement (65). Yan et al. reported a multicenter experience of 401 patients with the majority undergoing CRS and HIPEC for peritoneal mesothelioma, finding epithelial histologic subtype, absence of lymph node metastases, CC score and receipt of HIPEC therapy to be associated with survival (66). Similarly, Chua et al. reported improved outcomes for CRS HIPEC in patients with PMP of appendiceal origin when optimal cytoreduction was achieved (67). These cumulative multicentric studies, while retrospective, have provided important information for management of these diseases and have identified significant prognostic factors, which could help in the counseling and selection of patients for CRS/HIPEC surgery.
Looking ahead
Future developments in the evolution of HIPEC aim towards optimizing cancer-specific treatments on many levels including selection of patients, peri-operative care, and defining the safest and most effective chemotherapy regimens. The concept of bidirectional chemotherapy, administering concomitant IV and intraperitoneal chemotherapy in consecutive sessions, is currently under investigation (68). Moreover, research into new antineoplastic agents continues to expand in efficacy and directed therapy approaches. Immunotherapy is increasingly being used in the treatment of advanced malignancies and may eventually play a more important role specifically in the context of cancers with peritoneal dissemination. There remains variability in the methods of cytoreduction and HIPEC application and further studies will no doubt help to further standardize these techniques. CRS/HIPEC has evolved significantly over the past several decades and the future holds the promise of even more innovation and improvement.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Roviello F, Caruso S, Marrelli D, et al. Treatment of peritoneal carcinomatosis with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: state of the art and future developments. Surg Oncol 2011;20:e38-54. [PubMed]
- Meigs JV. Tumors of the female pelvic organs. New York: The Macmillan Co., 1934.
- Dembo AJ, Bush RS, Beale FA, et al. Ovarian carcinoma: improved survival following abdominopelvic irradiation in patients with a completed pelvic operation. Am J Obstet Gynecol 1979;134:793-800. [PubMed]
- Bruckner HW, Cohen CJ, Goldberg JD, et al. Improved chemotherapy for ovarian cancer with cis-diamminedichloroplatinum and adriamycin. Cancer 1981;47:2288-94. [PubMed]
- Munnell EW. The changing prognosis and treatment in cancer of the ovary. A report of 235 patients with primary ovarian carcinoma 1952-1961. Am J Obstet Gynecol 1968;100:790-805. [PubMed]
- Munnell EW. Surgical treatment of ovarian carcinoma. Clin Obstet Gynecol 1969;12:980-92. [PubMed]
- Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr 1975;42:101-4. [PubMed]
- Griffiths CT, Parker LM, Fuller AF Jr. Role of cytoreductive surgical treatment in the management of advanced ovarian cancer. Cancer Treat Rep 1979;63:235-40. [PubMed]
- Long RT, Spratt JS Jr, Dowling E. Pseudomyxoma peritonei. New concepts in management with a report of seventeen patients. Am J Surg 1969;117:162-9. [PubMed]
- Ghosh BC, Huvos AG, Whiteley HW. Pseudomyxoma peritonei. Dis Colon Rectum 1972;15:420-5. [PubMed]
- Dedrick RL, Myers CE, Bungay PM, et al. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep 1978;62:1-11. [PubMed]
- Pretorius RG, Petrilli ES, Kean CK, et al. Comparison of the iv and ip routes of administration of cisplatin in dogs. Cancer Treat Rep 1981;65:1055-62. [PubMed]
- Larkin JM, Edwards WS, Smith DE, et al. Systemic thermotherapy: description of a method and physiologic tolerance in clinical subjects. Cancer 1977;40:3155-9. [PubMed]
- Shingleton WW, Parker RT. Abdominal perfusion for cancer chemotherapy using hypothermia and hyperthermia. Acta Unio Int Contra Cancrum 1964;20:465-8. [PubMed]
- Spratt JS, Adcock RA, Sherrill W, et al. Hyperthermic peritoneal perfusion system in canines. Cancer Res 1980;40:253-5. [PubMed]
- Spratt JS, Adcock RA, Muskovin M, et al. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res 1980;40:256-60. [PubMed]
- Howell SB, Pfeifle CL, Wung WE, et al. Intraperitoneal cisplatin with systemic thiosulfate protection. Ann Intern Med 1982;97:845-51. [PubMed]
- Casper ES, Kelsen DP, Alcock NW, et al. Ip cisplatin in patients with malignant ascites: pharmacokinetic evaluation and comparison with the iv route. Cancer Treat Rep 1983;67:235-8. [PubMed]
- Zimm S, Cleary SM, Lucas WE, et al. Phase I/pharmacokinetic study of intraperitoneal cisplatin and etoposide. Cancer Res 1987;47:1712-6. [PubMed]
- Howell SB, Zimm S, Markman M, et al. Long-term survival of advanced refractory ovarian carcinoma patients with small-volume disease treated with intraperitoneal chemotherapy. J Clin Oncol 1987;5:1607-12. [PubMed]
- Colombo N, Speyer JL, Green M, et al. Phase II study of carboplatin in recurrent ovarian cancer: severe hematologic toxicity in previously treated patients. Cancer Chemother Pharmacol 1989;23:323-8. [PubMed]
- Sugarbaker PH. Surgical management of peritoneal carcinosis: diagnosis, prevention and treatment. Langenbecks Arch Chir 1988;373:189-96. [PubMed]
- Chu DZ, Lang NP, Thompson C, et al. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer 1989;63:364-7. [PubMed]
- Sugarbaker PH. Treatment of peritoneal carcinomatosis from colon or appendiceal cancer with induction intraperitoneal chemotherapy. Cancer Treat Res 1996;82:317-25. [PubMed]
- Koga S, Hamazoe R, Maeta M, et al. Treatment of implanted peritoneal cancer in rats by continuous hyperthermic peritoneal perfusion in combination with an anticancer drug. Cancer Res 1984;44:1840-2. [PubMed]
- Koga S. Prophylactic and therapeutic continuous hyperthermic peritoneal perfusion for peritoneal metastases of gastric cancer. Gan No Rinsho 1985;31:1103-5. [PubMed]
- Fujimoto S, Shrestha RD, Kokubun M, et al. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann Surg 1988;208:36-41. [PubMed]
- Toi M, Shiramizu T, Yonemura T, et al. Intraperitoneal cisplatin in peritoneal carcinomatosis patients. Gan No Rinsho 1985;31:522-6. [PubMed]
- Sugarbaker PH, Gianola FJ, Speyer JL, et al. Prospective randomized trial of intravenous v intraperitoneal 5-FU in patients with advanced primary colon or rectal cancer. Semin Oncol 1985;12:101-11. [PubMed]
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29-42. [PubMed]
- Horsell KW, Merten S, Clingan P, et al. Peritonectomy and intraperitoneal chemotherapy in appendiceal and colorectal cancer. Aust N Z J Surg 1999;69:729-32. [PubMed]
- Gilly FN, Beaujard A, Glehen O, et al. Peritonectomy combined with intraperitoneal chemohyperthermia in abdominal cancer with peritoneal carcinomatosis: phase I-II study. Anticancer Res 1999;19:2317-21. [PubMed]
- Glehen O, Cotte E, Kusamura S, et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol 2008;98:242-6. [PubMed]
- Sugarbaker PH, Yu W, Yonemura Y, et al. Gastrectomy, peritonectomy, and perioperative intraperitoneal chemotherapy: The evolution of treatment strategies for advanced gastric cancer. Semin Surg Oncol 2003;21:233-48. [PubMed]
- Stephens AD, Alderman R, Chang D, et al. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol 1999;6:790-6. [PubMed]
- Boutros C, Somasundar P, Espat NJ. Early results on the use of biomaterials as adjuvant to abdominal wall closure following cytoreduction and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol 2010;8:72. [PubMed]
- Jacquet P, Stuart OA, Chang D, et al. Effects of intra-abdominal pressure on pharmacokinetics and tissue distribution of doxorubicin after intraperitoneal administration. Anticancer Drugs 1996;7:596-603. [PubMed]
- Elias D, Antoun S, Goharin A, et al. Research on the best chemohyperthermia technique of treatment of peritoneal carcinomatosis after complete resection. Int J Surg Investig 2000;1:431-9. [PubMed]
- Rat P, Benoit L, Cheynel N, et al. Intraperitoneal chemo-hyperthermia with "overflow" open abdomen. Ann Chir 2001;126:669-71. [PubMed]
- Sugarbaker PH. An instrument to provide containment of intraoperative intraperitoneal chemotherapy with optimized distribution. J Surg Oncol 2005;92:142-6. [PubMed]
- Fujimura T, Yonemura Y, Fushida S, et al. Continuous hyperthermic peritoneal perfusion for the treatment of peritoneal dissemination in gastric cancers and subsequent second-look operation. Cancer 1990;65:65-71. [PubMed]
- Esquivel J, Averbach A. Laparoscopic Cytoreductive Surgery and HIPEC in Patients with Limited Pseudomyxoma Peritonei of Appendiceal Origin. Gastroenterol Res Pract 2012. [PubMed]
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359-74. [PubMed]
- Esquivel J, Farinetti A, Sugarbaker PH. Elective surgery in recurrent colon cancer with peritoneal seeding: when to and when not to proceed. G Chir 1999;20:81-6. [PubMed]
- Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol 2005;2:3. [PubMed]
- Sugarbaker PH. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol 1998;14:254-61. [PubMed]
- Elias D, Blot F, El Otmany A, et al. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer 2001;92:71-6. [PubMed]
- Bozzetti F, Yu W, Baratti D, et al. Locoregional treatment of peritoneal carcinomatosis from gastric cancer. J Surg Oncol 2008;98:273-6. [PubMed]
- Glockzin G, Schlitt HJ, Piso P. Peritoneal carcinomatosis: patients selection, perioperative complications and quality of life related to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol 2009;7:5. [PubMed]
- Gilly FN, Carry PY, Sayag AC, et al. Regional chemotherapy (with mitomycin C) and intra-operative hyperthermia for digestive cancers with peritoneal carcinomatosis. Hepatogastroenterology 1994;41:124-9. [PubMed]
- Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg 1981;11:127-39. [PubMed]
- Witkamp AJ, de Bree E, Kaag MM, et al. Extensive cytoreductive surgery followed by intra-operative hyperthermic intraperitoneal chemotherapy with mitomycin-C in patients with peritoneal carcinomatosis of colorectal origin. Eur J Cancer 2001;37:979-84. [PubMed]
- Glehen O, Gilly FN. Quantitative prognostic indicators of peritoneal surface malignancy: carcinomatosis, sarcomatosis, and peritoneal mesothelioma. Surg Oncol Clin N Am 2003;12:649-71. [PubMed]
- Cotte E, Passot G, Gilly FN, et al. Selection of patients and staging of peritoneal surface malignancies. World J Gastrointest Oncol 2010;2:31-5. [PubMed]
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737-43. [PubMed]
- Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284-92. [PubMed]
- Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358-63. [PubMed]
- Cotte E, Peyrat P, Piaton E, et al. Lack of prognostic significance of conventional peritoneal cytology in colorectal and gastric cancers: results of EVOCAPE 2 multicentre prospective study. Eur J Surg Oncol 2013;39:707-14. [PubMed]
- Fujimura T, Yonemura Y, Muraoka K, et al. Continuous hyperthermic peritoneal perfusion for the prevention of peritoneal recurrence of gastric cancer: randomized controlled study. World J Surg 1994;18:150-5. [PubMed]
- Rossi CR, Pilati P, Mocellin S, et al. Hyperthermic intraperitoneal intraoperative chemotherapy for peritoneal carcinomatosis arising from gastric adenocarcinoma. Suppl Tumori 2003;2:S54-7. [PubMed]
- Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008;15:2426-32. [PubMed]
- Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575-81. [PubMed]
- Klaver CE, Musters GD, Bemelman WA, et al. Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with colon cancer at high risk of peritoneal carcinomatosis; the COLOPEC randomized multicentre trial. BMC Cancer 2015;15:428. [PubMed]
- Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 2010;28:63-8. [PubMed]
- Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009;27:6237-42. [PubMed]
- Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012;30:2449-56. [PubMed]
- Bijelic L, Stuart OA, Sugarbaker P. Adjuvant bidirectional chemotherapy with intraperitoneal pemetrexed combined with intravenous Cisplatin for diffuse malignant peritoneal mesothelioma. Gastroenterol Res Pract 2012. [PubMed]


