Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer: survival outcomes and patient selection
Introduction
Peritoneal metastases (PM) are a frequent manifestation in the natural history of colorectal cancer (CRC) and are associated with limited survival (1). About 8% of patients at the time of primary resection, and up to 25% of patients with recurrent CRC will develop metastatic disease confined to the peritoneal surfaces (2). When it comes to treatment of patients with CRC with PM (CRC-PM), there appears to be a dichotomy that unfortunately continues to grow deeper roots: more than 90% of patients will be treated with a combination of palliative cytotoxic chemotherapy and a biological agent and about 5% will be treated with a combined modality that incorporates cytoreductive surgery (CRS) to remove all visible metastatic disease to the peritoneal cavity and hyperthermic intraperitoneal chemotherapy (HIPEC) to eradicate microscopic residual disease. When it comes to published outcomes from these treatment modalities, the exact opposite can be found: the vast majority of the literature reflects outcomes from CRS and HIPEC and very few studies report on the outcome of patients treated with systemic therapies. Some of the cited reasons for the low inclusion of CRC-PM patients into clinical trials include: (I) mixing all patients with stages IV A and B; (II) relative low incidence (less than 20%) of PM; (III) PM are usually associated with other sites of metastases; and (IV) patients with low tumor burden are difficult to be evaluated with RECIST criteria (3). When it comes to selection criteria for treatment type and sequence of therapeutic modalities at the time of diagnosis of PM of colorectal origin, the selection criteria for either treatment strategy remain ill-defined. In addition, there is no established non-surgical process to rationally select patients for management, either for inclusion/stratification in clinical trials or as a component of standard-of-care (4). Consequently, precise pre-treatment stratification represents an unmet need in oncology.
The aim of this study is to review outcome data from both treatment modalities and to present a clinical pathway that incorporates all currently available therapies, determines the sequence and duration of these therapies from the time of diagnosis of PM from CRC and establishes selection criteria based on the existing evidence and published outcomes.
Materials and methods
We conducted a comprehensive literature search of PubMed using the words CRC with PM and focused on manuscripts from 2004 to 2015 that included data on selection criteria and outcomes from either of the two treatment modalities (systemic therapies or CRS and HIPEC) as well as any publications that incorporated in a predetermined fashion the timing and sequence of such therapies. We also included manuscripts that focused on trying to determine a different combination of the available therapies and some of the ongoing clinical trials. Based on an analysis of existing evidence, we constructed a clinical pathway that starts at the time of diagnosis of PM of colorectal origin with precise non-surgical pretreatment stratification and that incorporates all currently available therapies, determining the sequence and timing of such therapies.
Results
Outcome of patients treated with systemic therapies
Even though many prospective randomized trials have been conducted in patients with unresectable, metastatic CRC, very few of them have included patients with metastatic disease that involves the peritoneum. Analysis of four studies from both sides of the Atlantic (5-7) demonstrates that few patients with PM of colorectal origin are included in these trials and that the vast majority of these patients will also have other sites of hematogenous metastases.
First, Franko and colleagues (5) reported a pooled analysis of two large phase III trials from the North Central Cancer Treatment Group (NCCTG) that included 2,101 patients with CRC-PM treated only with systemic chemotherapy. Of these 2,101 patients, 1,646 patients participated in the N9741 trial that evaluated first-line chemotherapy for metastatic CRC. The remaining participated in the N9841 trial that evaluated the role of second-line chemotherapy (n=455). Over 80% of these patients had metastatic disease via the hematogenous route and just 17% of the total group had PM in addition to liver and/or lung metastases. Forty-four patients, 2.1%, had metastatic disease confined to the peritoneal surfaces.
Evaluation of their outcome demonstrated that patients with PM had higher risk of death owing to all causes than patients without PM (median OS, 12.7 vs. 17.6 months; HR, 1.32; 95% CI, 1.15-1.50; P<0.001). This unfavorable prognostic influence of PM, persisted even after adjusting for age, performance status, liver metastases, and other factors (OS: HR, 1.3; P<0.001).
Two similar studies, CAIRO and CAIRO 2, conducted by the Dutch Colorectal Cancer Group (DCCG) (6) were reported by Klaver and colleagues. In the CAIRO study (7), 820 patients were randomized between sequential treatment (first-line: capecitabine, second-line: irinotecan, and third-line: oxaliplatin plus capecitabine, arm A) and combination treatment (first-line: irinotecan plus capecitabine, second-line: oxaliplatin plus capecitabine, arm B). In the CAIRO2 study (8), 755 patients were randomized between capecitabine, oxaliplatin, and bevacizumab (CB regimen), and the same regimen plus weekly cetuximab (CBC regimen).
An analysis of the type of patients enrolled in these two phase III studies showed similar results as the North American studies, with over 90% of the patients having no evidence of metastatic disease involving the peritoneal surfaces. In the CAIRO study only 34 patients (4%) had PM and of these 34 patients only 4 had isolated PM. In the CAIRO2 study, only 47 patients (6%) had PM and 5 of them had isolated PM.
An analysis of the outcome on both studies showed that the presence of PM was associated with a decreased survival when compared to those patients without PM. Median OS in the CAIRO study were 10.4 months versus 17.3 months for patients with and without PM (P<0.001). Similar results were found on CAIRO2, with median OS of 15.2 versus 20.7 months (P<0.001). Interestingly, the median number of treatment cycles between patients with or without PM did not differ in both studies. However, the occurrence of major toxicity was more frequent in patients with PM treated with sequential chemotherapy in the CAIRO study as compared to patients without PM but this was not reflected in reasons to discontinue treatment. No difference in major toxicity was observed in the CAIRO2 study.
The authors concluded that these findings demonstrate a decreased efficacy of current standard chemotherapy with or without biological agents in patients with PM of colorectal origin when compared to those patients without PM. In addition, they concluded that this difference cannot be explained by undertreatment or increased susceptibility to toxicity, but rather that there most exist a different biological behavior of tumors that spread to the peritoneal cavity that conveys a relative resistance to treatment.
On another report, Chua and colleagues reviewed the therapeutic options of 2,492 patients with metastatic colon cancer from 19 studies between 1995 and 2009. He reported a survival of only 12.5 months [5-24] for patients having undergone palliative surgery and/or systemic chemotherapy versus a survival of 33 months [20-63] for patients that underwent a more comprehensive treatment strategy with a complete CRS and HIPEC (9).
Outcome of patients treated with CRS and HIPEC
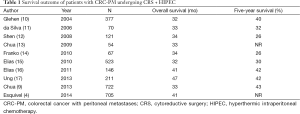
Table 1 includes a summary of recent publications from centers around the world that include at least 50 patients on their studies, treated with CRS and HIPEC. It is interesting to see that median survival of almost 3 years is very common with a few studies reporting median survivals of 40 plus months, with median 5-year survivals of about 30%. The common denominator for a good long term result includes achieving a complete cytoreduction and avoiding surgery in patients with large tumor burden and poorly differentiated/signet ring cell histologies.
Full table
Prodige 7
Prodige 7 is a prospective randomized multicenter phase III trial by the French group where patients with CRC and limited peritoneal dissemination were taken to the operating room. The study was designed to evaluate what is the added benefit of HIPEC to a complete CRS (18). If a complete CRS was achieved, the patients were randomized in the operating room to receive HIPEC or not. This study finished accrual at the end of 2013 and we are anxiously awaiting the results. In this study, HIPEC was delivered with oxaliplatin (460 mg/m2) in 2 L/m2 of dextrose 5% over 30 minutes at a minimal temperature of 42 °C. One hour before the HIPEC, 20 mg/m2 of leucovorin and 400 mg/m2 of 5-fluorouracil were given intravenously.
Outcomes after complete cytoreductive surgery (CRS) and systemic therapy only
Recently, Desoineux et al. (19) reported a very interesting manuscript. They recognize that although the efficacy of surgery in patients with CRC-PM has been demonstrated, the evidence to support the role of HIPEC is less certain. To address this issue, they reported the overall survival (OS), progression-free survival (PFS) and morbidity on fifty consecutively included patients treated for CRC-PM with complete CRS and systemic chemotherapy only.
The median peritoneal cancer index (PCI) was 8 (range, 1-24). Twenty three patients had liver or lung metastases (LLM). Twenty two patients had synchronous metastases. Median follow-up was 62.5 months (95% CI, 45.4-81.3) and median survival was 32.4 months (21.5-41.7). Three- and 5-year OS were 45.5% (0.31-0.59) and 29.64% (0.17-0.44) respectively. Presence of LLMs with PM was significantly associated with poorer prognosis, with survival at 5 years of 13.95% (95% CI, 2.9-33.6) vs. 43.87% (22.2-63.7) when no LLM were present (P=0.018). Median PFS was 9.5 months (95% CI, 6.2-11.1).
They concluded that with an equivalent PCI range and despite one of the highest rates of LLM in the literature, their survival data of CRS + systemic chemotherapy only compare well with results reported after additional HIPEC. The therapy was well tolerated with acceptable morbidity without any mortality.
Clinical pathway
Figure 1 represents a clinical pathway for the suggested management of patients with CRC-PM from the time of diagnosis of their PM. The pathway incorporates all currently available therapies and stratifies patients by the Peritoneal Surface Disease Severity Score (PSDSS).
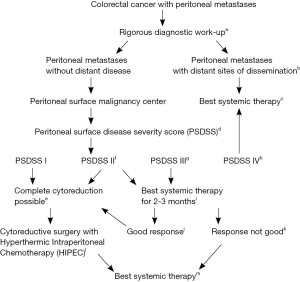
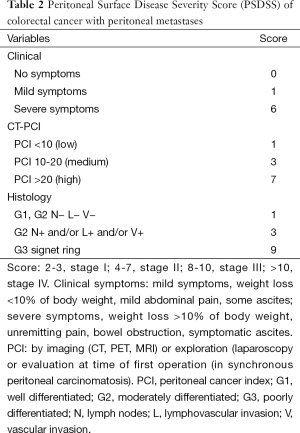
Full table
Discussion
Individualized, sometimes even personalized, based on genomic profile analyses, multidisciplinary care is the hallmark of cancer care in 2015. Unfortunately, due to reasons that are hard to decipher, this is not the case for patients with CRC with PM. The vast majority of them, over 90% in the United States, are being treated in a generic and mono-disciplinary fashion by medical oncologists with the strategy of continuing combinations of cytotoxic chemotherapy with biological/targeted agents until (I) disease progression; (II) intolerable side effects; or (III) death. The 2015 NCCN guidelines (21) include recommendations for a multidisciplinary evaluation of patients with CRC metastatic to the liver and/or lung, including the evaluation by a thoracic and/or liver surgeon but if the patients have PM, the recommendations switch to generic and mono-disciplinary, including only palliative systemic therapy. This strategy has proven beneficial to many patients with unresectable metastatic CRC with outcomes that over the last 20 years have gone from a median survival of 12 to now 30 months. This strategy is also the result of prospective randomized trials that include multiple institutions with large number of patients. However, the majority of these patients have liver and/or lung metastases and very few of the patients entered into these clinical trials will have PM. Some of the reasons for these low numbers include the fact that PM are difficult to be characterized with current imaging modalities and the fact that many patients with CRC-PM also have other sites of metastatic disease. Consequently, the outcome of patients with isolated PM treated with modern systemic therapies remains unknown for the most part and the strategy of treatment is extrapolated from a different group of patients: patients with multiple sites of metastases that are not candidates for surgery.
As shown on Table 1, multiple studies demonstrate a very favorable median survival when CRS and HIPEC are incorporated into the treatment algorithm of these patients. However, these studies are retrospective studies and include highly selected patients with most of them having received many cycles of systemic therapy. In addition, the question of how much does HIPEC contribute to a complete cytoreduction is being asked with an increased frequency but remains unanswered. Also, the selection criteria for CRS and HIPEC continue to be ill-defined. This might be in part because selection criteria are a process in evolution. Traditionally, we have focused on selection criteria that could help us identify those patients that could have a complete cytoreduction. Over the next years, we realized that not all patients with a complete cytoreduction derived long term benefit (22). We started adding the role of tumor burden and histology as well as clinical symptoms in the PSDSS. This score demonstrated that the outcome of patients with CRC-PM undergoing a complete CRS and HIPEC is much more complex than achieving a complete cytoreduction.
The French trial (Prodige 7) was designed to evaluate what is the added benefit of HIPEC to a complete CRS. The participation of multiple institutions with varying degrees of experience and the fact that the timing of incorporation of systemic therapies and the agents used were not mandated to participate on the trial, will make this trial in my estimate, a negative study that will fail to demonstrate the role of HIPEC. In addition, it is possible that patients that randomized to the no HIPEC arm, might receive another surgery with HIPEC after they recur. Having said that, a very important contribution will be that it will show the value of having surgery to remove the PM and receiving systemic chemotherapy. Therefore, this will be a landmark study highlighting the importance of a multidisciplinary management of patients with CRC-PM.
In 2015, much of our efforts should be directed at trying to establish precise-pretreatment stratifying parameters that will help us evaluate the role of all currently available therapies and will assist in identifying relative and absolute prognostic indicators that can be the basis of prospective trials. A very important study would be to evaluate the role of systemic therapies in patients with isolated CRC-PM and stratified by the PSDSS. This study is being carried out in the United States every day in many patients but nobody is keeping track of it. There is no reason why medical and surgical oncologists should not be able to work together and offer a true multidisciplinary evaluation to all patients with PM (23). Entering these patients into prospective registries is a necessary first step that can happen today.
Our future goal should be to increase the resectability of patients with CRC-PM by improving selection criteria and early referrals but also by using systemic therapies in a neo-adjuvant setting. Better outcomes will be tied to therapies that help to maintain the complete surgical response and whether that includes HIPEC and/or more systemic therapies will have to be determined in due time.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358-63. [PubMed]
- American Cancer Society. Cancer Facts & Figures. 2012. Available online: http://www.cancer.org
- Chan CH, Cusack JC, Ryan DP. A critical look at local-regional management of peritoneal metastasis. Hematol Oncol Clin North Am 2015;29:153-8. [PubMed]
- Esquivel J, Lowy AM, Markman M, et al. The American Society of Peritoneal Surface Malignancies (ASPSM) Multiinstitution Evaluation of the Peritoneal Surface Disease Severity Score (PSDSS) in 1,013 Patients with Colorectal Cancer with Peritoneal Carcinomatosis. Ann Surg Oncol 2014;21:4195-201. [PubMed]
- Franko J, Shi Q, Goldman CD, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol 2012;30:263-7. [PubMed]
- Klaver YL, Simkens LH, Lemmens VE, et al. Outcomes of colorectal cancer patients with peritoneal carcinomatosis treated with chemotherapy with and without targeted therapy. Eur J Surg Oncol 2012;38:617-23. [PubMed]
- Koopman M, Antonini NF, Douma J, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet 2007;370:135-42. [PubMed]
- Tol J, Koopman M, Rodenburg CJ, et al. A randomised phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first-line advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). An interim analysis of toxicity. Ann Oncol 2008;19:734-8. [PubMed]
- Chua TC, Esquivel J, Pelz JO, et al. Summary of current therapeutic options for peritoneal metastases from colorectal cancer. J Surg Oncol 2013;107:566-73. [PubMed]
- Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284-92. [PubMed]
- da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg 2006;203:878-86. [PubMed]
- Shen P, Thai K, Stewart JH, et al. Peritoneal surface disease from colorectal cancer: comparison with the hepatic metastases surgical paradigm in optimally resected patients. Ann Surg Oncol 2008;15:3422-32. [PubMed]
- Chua TC, Yan TD, Ng KM, et al. Significance of lymph node metastasis in patients with colorectal cancer peritoneal carcinomatosis. World J Surg 2009;33:1488-94. [PubMed]
- Franko J, Ibrahim Z, Gusani NJ, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer 2010;116:3756-62. [PubMed]
- Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 2009;27:681-5. [PubMed]
- Elias D, Honoré C, Dumont F, et al. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg 2011;254:289-93. [PubMed]
- Ung L, Chua TC, David LM. Peritoneal metastases of lower gastrointestinal tract origin:a comparative study of patient outcomes following cytoreduction and intraperitoneal chemotherapy. J Cancer Res Clin Oncol 2013;139:1899-1908. [PubMed]
- Elias D, Goéré D, Dumont F, et al. Role of hyperthermic intraoperative peritoneal chemotherapy in the management of peritoneal metastases. Eur J Cancer 2014;50:332-40. [PubMed]
- Désolneux G, Mazière C, Vara J, et al. Cytoreductive surgery of colorectal peritoneal metastases: outcomes after complete cytoreductive surgery and systemic chemotherapy only. PLoS One 2015;10:1-12. [PubMed]
- Esquivel J, Elias D, Baratti D, et al. Consensus statement on the loco regional treatment of colorectal cancer with peritoneal dissemination. J Surg Oncol 2008;98:263-7. [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Colon/Rectal Cancer 2015. Available online: www.nccn.org
- Pelz JO, Stojadinovic A, Nissan A, et al. Evaluation of a peritoneal surface disease severity score in patients with colon cancer with peritoneal carcinomatosis. J Surg Oncol 2009;99:9-15. [PubMed]
- Esquivel J. Colorectal Cancer With Peritoneal Metastases: A Plea for Cooperation Between Medical and Surgical Oncologists. Oncology (Williston Park) 2015;29:521-2. [PubMed]


