Peritoneal dissemination from high-grade appendiceal cancer treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC)
Introduction
Appendiceal neoplasms are rare malignancies usually found in approximately 1% of appendectomy procedures (1,2). Although unusual, the incidence of appendiceal cancer is rising (3). Unfortunately, some appendiceal cancer patients go on to develop peritoneal surface malignancies (“carcinomatosis”), also known as mucinous carcinoma peritonei (MCP), or pseudomyxoma peritonei (PMP). Carcinomatosis results from peritoneal dissemination from a range of gastrointestinal cancers, most commonly appendiceal and colorectal cancers, as well as ovarian carcinomas, sarcomas, and mesotheliomas (4-10). In patients with peritoneal surface disease from high-grade appendiceal cancer, malignant ascites is a common finding, and is associated with a life expectancy ranging from weeks to a few months (11). During the last two decades, an increasing body of evidence in support of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) as a promising treatment for MCP/PMP. This aggressive approach is being utilized at a growing number of centers. However, it is most commonly applied to the peritoneal dissemination of low-grade appendiceal tumors (2,10), citing a 10-year survival rate as high as 63% (12). Importantly, within the subset of appendiceal tumors a spectrum of histologies affect a patient’s clinical outcome.
Traditionally, carcinomatosis has been approached with nihilism, and the optimal approach for PSD from high-grade appendiceal cancer is unclear; therefore, the optimal management of patients with PSD remains a matter of debate. While a few groups have reported treating PSD from appendiceal neoplasms with debulking alone (13-15), these procedures do not address intracavitary residual microscopic tumor. Similarly, laparoscopic HIPEC without CRS has been shown to offer palliation of malignant ascites, but leaves tumor intact (11,16) Several reports have linked systemic chemotherapy prior to CRS-HIPEC with poorer survival outcomes (2,12,17) CRS-HIPEC is the only treatment modality which addresses both the bulk tumor burden, and residual or microscopic disease.
One of the most important prognostic factors, in patients with PSD from appendiceal primaries, is histologic grade (18). Other prognostic factors affecting long-term survival include tumor biology, age, functional status, presence of nodal metastases and extent of disease at the time of diagnosis. It is important to note that not every patient with PSD from appendiceal tumors is associated with long-term survival, nor is every appendiceal primary associated with mucinous ascites (PMP). Despite a lack of proven efficacy, systemic chemotherapy is often recommended to patients with poor prognostic factors (high-grade histology, incomplete cytoreduction, lymph node involvement, and early recurrence). Such systemic chemotherapy is typically extrapolate from the considerable experience with colorectal cancer.
Our approach to peritoneal dissemination from high-grade appendiceal tumors has been aggressive CRS and HIPEC, with the goal of complete cytoreduction, if feasible. As we have previously reported, CRS-HIPEC has the potential to offer improved overall survival (OS) for select patients with MCP/PMP (2). The primary aim of this study was to evaluate disease-free and overall survival outcomes in patients with PSD from high-grade appendiceal carcinoma who underwent CRS-HIPEC at our institution between 1991 and 2015. Specifically, we aimed to identify the impact of complete CRS in this population when compared to high-grade colon cancer.
Methods
Using a prospectively maintained database we retrospectively examined 1,223 CRS-HIPEC procedures performed between 12/30/91 and 01/22/15. Institutional Review Board approval was obtained for this study. Initial selection included patients with a histologic or cytologic diagnosis of peritoneal surface malignancy, with a final pathologic diagnosis of high-grade primary appendiceal or colon cancer. Of note, presence of signet-ring cells, or any poorly differentiated lesion, was considered high-grade. Neuroendocrine tumors with or without goblet-cell features were included. Patients were analyzed on the basis of demographics, age, race, gender, Eastern Cooperative Oncology Group (ECOG) performance status, extent of resection (R-status), nodal status, use of preoperative chemotherapy, and volume of peritoneal disease (PCI score). Morbidity, mortality, and survival were also reviewed. Preoperative systemic chemotherapy was defined as any systemic therapy received within three months prior to CRS-HIPEC.
In general, the eligibility criteria for CRS-HIPEC included patients who are medically fit, with an ECOG performance status ≤2, without extraabdominal disease. The peritoneal disease had to be debulkable, with a resectable or resected primary lesion. If parenchymal hepatic metastases were present they must be easily resectable or ablatable without lobar hepatectomy. All patients were evaluated preoperatively, including: a complete history and physical exam, blood counts, renal and liver function tests, tumor markers, computed tomography of the chest, abdomen and pelvis (CT C/A/P) or magnetic resonance imaging (MRI), as well as a pathologic review of prior specimens and/or cytology.
All selected patients underwent the CRS-HIPEC procedure as described previously by our group (16,19). Patients deemed appropriate for CRS-HIPEC were explored via midline incision. Aggressive cytoreduction of involved visceral organs and peritoneal surfaces was performed with peritonectomy procedures performed only for apparent disease. The omentum was routinely removed. Two inflow and outflow catheters are placed, and the abdomen is temporarily closed while either: 40 mg/m2 mitomycin-C (MMC) or 200 mg/m2 oxaliplatin is added to the perfusate. The heat is titrated to an inflow temperature of 40 to 42 °C while maintaining an outflow temperature of 40 °C. The chemotherapeutic agent is circulated through the peritoneal cavity for 120 minutes as per protocol. Afterward, the perfusate is drained, the skin re-opened, and the abdomen inspected a final time before being definitively closed. Surgical morbidity and mortality were recorded according to the Clavien-Dindo Classification system (20). The completeness of resection was graded by the surgeon at the conclusion of the procedure according to AJCC staging guidelines: R0/1—complete cytoreduction of all visible disease; R2a—minimal residual disease, nodules <0.5 cm; R2b—gross residual disease, nodules between 0.5 and 2.0 cm; and R2c—extensive residual disease, nodules >2.0 cm. Postoperatively, patients were followed every 6 months for the first 3 years and then annually through year 5 with physical exam, tumor markers, and imaging to monitor for disease recurrence or progression.
All data were prospectively collected and retrospectively analyzed. Descriptive statistics were reported for continuous and categorical variables including frequencies and percentages for categorical data, and means, standard deviations, medians and ranges for continuous data. Disease-free survival (DFS) and OS were calculated as the interval between the date of CRS-HIPEC (not the date of diagnosis) and the date of last known follow-up or the date of death. In cases whereby a patient underwent more than one CRS-HIPEC, OS was calculated from the date of the first procedure. Estimates of survival were calculated by using the Kaplan-Meier (product limit) method, and survival estimates were compared using the log-rank test. Statistical significance was defined as a P value <0.05. All analyses were performed using SAS 9.4 (SAS, Cary, NC, USA).
Results
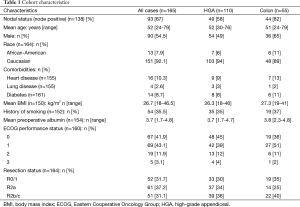
A review of 1,223 CRS-HIPEC procedures, between 12/30/91 and 01/22/15, identified 171 surgeries (165 patients) performed for high-grade appendiceal and high-grade colon cancers. Of those procedures, 110 (66.7%) were performed for HGA and 55 (33.3%) for colorectal lesions. Six patients underwent CRS-HIPEC twice with repeat procedures being excluded from statistical analyses. The median follow-up period for the entire cohort was 42 months. The demographic details are presented in Table 1.
Full table
Patient characteristics
The mean age of the 165 patients with HGA and high-grade colon cancer who underwent CRS-HIPEC during the study period was 51.8 years, see Table 1. Eastern Cooperative Oncology Group (ECOG) performance status was also favorable with 85% of patients having an ECOG status of 0 or 1. Lymph node data were available for 138 patients with 67.4% of these having lymph node metastases at the time of CRS-HIPEC. Prior to surgery, 55.8% of the cohort received preoperative chemotherapy. The mean PCI in all patients was 16.9 (range, 2-34). The agent used at HIPEC was mitomycin-C in 143 (86.7%) patients, and oxaliplatin in 22 (13.3%). Complete cytoreduction (R0/R1) was accomplished in 52 (31.7%) patients, with 61 (37.2%) receiving an R2a resection, and 51 (31.1%) an R2b/c resection. Forty-four patients (64.7%) received systemic chemotherapy after CRS-HIPEC. The median follow-up time was 42 months.
Morbidity and mortality
The 30-day combined Clavien-Dindo I/II minor morbidity rates were 44.1%, while the 30-day major morbidity rates were 25.5%. Postoperatively, patients spent an average of 3.8 days (SD 10.7) in the intensive care unit (ICU), the median was 1 day, with 16.6 days hospitalized (SD 19.8), and a median 10 days. The 30-day mortality rate for the entire cohort was 3.6%. Thirty-one patients (29.0%) were readmitted within 30 days of discharge. The morbidity and mortality details are presented in Table 2.

Full table
Survival
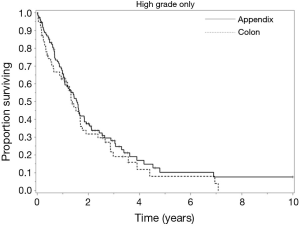
Overall DFS demonstrated a median of 14.4 months, compared to the observed OS of 18 months. Patients with R0/R1 complete macroscopic cytoreduction had a survival rate significantly better than patients with R2a or R2b/c resections (respective medians of: 36, 15.6, and 8.4 months); P<0.0001) (Figure 1). Interestingly, patients who underwent preoperative chemotherapy prior to CRS-HIPEC exhibited significantly poorer OS compared to patients who did not receive systemic treatment (respective medians of: 14.4 and 20.4 months; P=0.01). Completeness of cytoreduction and preoperative chemotherapy administration were not statistically significant when measuring DFS.
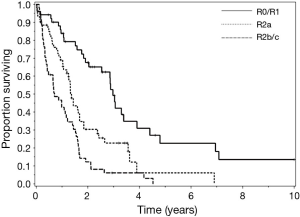
Univariate analyses found that significant predictors of survival were; R-status (R0/R1 36 months, R2a 15.6 months, and R2b/c 8.4 months; P<0.0001), nodal status (negative 39.6 months and positive 15.6 months; P<0.0001), ECOG performance status (P=0.0015), the number of organs resected (P=0.0006), the administration of preoperative chemotherapy (20.4 vs. 14.4 months; P=0.01), and postoperative chemotherapy (4.8 vs. 34.8 months; P<0.0001). Of 159 cases 54 had high-grade adenocarcinoma histology with no evidence of signet ring or goblet cell features; 66 were classified as adenocarcinoid, and 39 exhibited signet ring cells. Despite the stratification of high-grade histologic features, no statistical significance was observed in the single-variable model when comparing survival (P=0.25).
In the multivariate models, fitted using variables with a univariate P value less than 0.025, R-status, nodal status, ECOG, and the administration of preoperative chemotherapy continued to be statistically significant predictors of survival in a full model. However, in the reduced multivariate model, fitted in an iterative fashion by removing variables with P values >0.05, until only those with a P value less than 0.05 remained, only the completeness of resection and nodal status showed significance for survival in patients with high-grade disease.
Discussion
Despite high-grade tumor biology PSD from appendiceal and colorectal primary lesions can be treated with CRS-HIPEC in select patients. While tumor grade and operative intervention feasibility remain strong determinants of survival outcomes in patients with carcinomatosis, our data exhibit acceptable morbidity and mortality rates for patients with stage IV disease, despite significant disease burden and aggressive biology at presentation (4,16). Further, complication rates and length of stay (LOS) was essentially identical for high grade appendiceal and colonic cancer patients undergoing HIPEC.
The similarity of the overall and disease free survival of the HGA and colorectal groups is striking. The HGA have long term survival rates of approximately 1/4-1/3 of that experienced by low grade appendiceal patients with similar completeness of cytoreduction. The most important prognostic indicators identified in patients with HGA and high-grade colon cancer were the completeness of cytoreduction and nodal status. These findings are in agreement with prior publications which demonstrate long-term survival is possible if R0/R1 resection is obtained (19,21-23).
In the setting of complete cytoreduction, lymph node involvement remained a significant prognostic value for survival emphasizing the role of tumor biology in predicting outcome. Clearly, nodal metastasis remains an important prognostic marker even in the presence of distant (peritoneal) metastases. As reported previously by our group, node-negative HGA and colorectal patients can achieve survival comparable with R0/R1 resected low-grade appendiceal primary lesions if diagnosed early and promptly referred for CRS-HIPEC when complete macroscopic cytoreduction is feasible (21). This makes the use of adjuvant chemotherapy in those with node-positive, high-grade disease imperative. Further, univariate analysis suggests that preoperative chemotherapy has better outcomes in this setting.
It has been shown that the administration of preoperative chemotherapy in HGA and high-grade colorectal primary lesions is associated with a significant decrease in OS (2,12,21,24).
This would seem to be related to a selection and/or referral bias. However, a lead time bias is another possible explanation for this difference. Therefore, for patients with low-volume disease (PCI<10), CRS-HIPEC could be attempted first, followed by systemic adjuvant chemotherapy, which would eliminate a lead time bias for these patients (10,21). For patients with higher-volume disease (PCI, 10-18), our group recommends upfront systemic chemotherapy and prompt reevaluation for surgery. We currently endeavor not operate on patients with high-grade, high-volume disease (PCI >20) unless they have received preoperative chemotherapy, with responsive or at least stable disease, and without measurable evidence of progression via serial imaging and tumor markers (4,10,21).
Most of the data regarding CRS and HIPEC has been garnered from low-grade appendiceal cancer with PSD. There are clearly substantial differences between the low-grade and high-grade lesions in terms of outcome, which highlights the importance of expert pathologic opinion when planning therapy and evaluating prognosis. Like the low-grade lesions, HGA should be treated with CRS preferably with HIPEC. However, HGA should also be treated with systemic chemotherapy in addition to surgery. We currently treat all, but low CT-PCI score patients, with preoperative chemotherapy, and preferably via an oxaliplatin based regimen (24-26).
Conclusions
In patients with PSD from HGA or high-grade colon primary lesions, early diagnosis and prompt referral for CRS-HIPEC is essential to optimizing outcomes, especially in those with low-volume disease (PCI <10) (2,4,10). Measures clinicians can use to identify patients who would benefit the most from CRS-HIPEC include PCI and nodal status. CT-PCI, in some cases supported by a laparoscopic exploration can be used to identify patients whose disease is not amenable to complete cytoreduction. Nodal status can be used to indicate a biologically aggressive disease, and node positive patients should clearly receive systemic therapy in addition to cytoreduction and HIPEC (4). The completeness of cytoreduction and lymph node involvement remain the most significant prognosticators for long-term survival in patients with PSD from HGA or high-grade colorectal primary lesions. The striking similarity of our outcomes for HGA and high-grade colon cancer suggests similar oncogenesis and supports the use of similar systemic therapy (Figure 2).
Acknowledgements
Funding: Smith Family foundation and the Comprehensive Cancer Center of Wake Forest University biostatistics shared resource supported by NCI CCSG P30CA012197.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Collins DC. 71,000 human appendix specimens. A final report, summarizing forty years' study. Am J Proctol 1963;14:265-81. [PubMed]
- Blackham AU, Swett K, Eng C, et al. Perioperative systemic chemotherapy for appendiceal mucinous carcinoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Oncol 2014;109:740-5. [PubMed]
- Marmor S, Portschy PR, Tuttle TM, et al. The rise in appendiceal cancer incidence: 2000-2009. J Gastrointest Surg 2015;19:743-50. [PubMed]
- Randle RW, Griffith KF, Fino NF, et al. Appendiceal goblet cell carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Res 2015;196:229-34. [PubMed]
- Yan TD, Welch L, Black D, et al. A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignancy peritoneal mesothelioma. Ann Oncol 2007;18:827-34. [PubMed]
- Yan TD, Black D, Savady R, et al. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol 2007;14:484-92. [PubMed]
- Yan TD, Black D, Savady R, et al. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol 2006;24:4011-9. [PubMed]
- Yonemura Y, Bando E, Kawamura T, et al. Cytoreduction and intraperitoneal chemotherapy for carcinomatosis from gastric cancer. Cancer Treat Res 2007;134:357-73. [PubMed]
- Blackham AU, Levine EA. Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Malignant Peritoneal Mesothelioma. European J Clin Med Oncol 2012;4:25-32. [PubMed]
- Ahmed S, Stewart JH, Shen P, et al. Outcomes with cytoreductive surgery and HIPEC for peritoneal metastasis. J Surg Oncol 2014;110:575-84. [PubMed]
- Randle RW, Swett KR, Swords DS, et al. Efficacy of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in the management of malignant ascites. Ann Surg Oncol 2014;21:1474-9. [PubMed]
- Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012;30:2449-56. [PubMed]
- Miner TJ, Shia J, Jaques DP, et al. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg 2005;241:300-8. [PubMed]
- Gough DB, Donohue JH, Schutt AJ, et al. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg 1994;219:112-9. [PubMed]
- Hinson FL, Ambrose NS. Pseudomyxoma peritonei. Br J Surg 1998;85:1332-9. [PubMed]
- Levine EA, Stewart JH 4th, Shen P, et al. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg 2014;218:573-85. [PubMed]
- Baratti D, Kusamura S, Nonaka D, et al. Pseudomyxoma peritonei: clinical pathological and biological prognostic factors in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol 2008;15:526-34. [PubMed]
- Votanopoulos KI, Shen P, Stewart JH 4th, et al. Current status and future directions in appendiceal cancer with peritoneal dissemination. Surg Oncol Clin N Am 2012;21:599-609. [PubMed]
- Levine EA, Stewart JH 4th, Russell GB, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg 2007;204:943-53; discussion 953-5. [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [PubMed]
- Votanopoulos KI, Russell G, Randle RW, et al. Peritoneal surface disease (PSD) from appendiceal cancer treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): overview of 481 cases. Ann Surg Oncol 2015;22:1274-9. [PubMed]
- El Halabi H, Gushchin V, Francis J, et al. The role of cytoreductive surgery and heated intraperitoneal chemotherapy (CRS/HIPEC) in patients with high-grade appendiceal carcinoma and extensive peritoneal carcinomatosis. Ann Surg Oncol 2012;19:110-4. [PubMed]
- Halabi HE, Gushchin V, Francis J, et al. Prognostic significance of lymph node metastases in patients with high-grade appendiceal cancer. Ann Surg Oncol 2012;19:122-5. [PubMed]
- Sugarbaker PH, Bijelic L, Chang D, et al. Neoadjuvant FOLFOX chemotherapy in 34 consecutive patients with mucinous peritoneal carcinomatosis of appendiceal origin. J Surg Oncol 2010;102:576-81. [PubMed]
- Shapiro JF, Chase JL, Wolff RA, et al. Modern systemic chemotherapy in surgically unresectable neoplasms of appendiceal origin: a single-institution experience. Cancer 2010;116:316-22. [PubMed]
- Eng C, Blackham A, Levine EA, et al. Systemic chemotherapy in the setting of unresectable appendiceal epithelial neoplasms (AEN). J Clin Oncol 2012;30:abstr 568.