Hyperthermic intraperitoneal chemotherapy for epithelial ovarian cancers: is there a role?
Introduction
Epithelial ovarian cancer (EOC) is the leading cause of death from gynecologic malignancies and the fifth most common cause of cancer death in women. In 2015, it is estimated that there will be 21,980 new cases of ovarian cancer with 14,270 deaths from this disease (1). Currently, there are no effective screening strategies and over 60% of patients present with stage III or IV disease (2). Management of patients with advanced EOC includes aggressive surgical cytoreduction followed by platinum-based chemotherapy (3,4). Between 70% and 80% of patients will achieve clinical remission; however, most patients will relapse and are not curable (5). Multiple advancements have occurred over the last several decades for women with EOC including: the incorporation of taxanes into chemotherapy regimens, the introduction of intraperitoneal (IP) and dose-dense treatment regimens, and the development of biologic agents such as bevacizumab and olaparib (4,6-9). Three Gynecologic Oncology Group (GOG) phase III clinical trials have demonstrated a significant improvement in both progression-free (PFS) and overall survival (OS) in the primary treatment of those patients with optimally cytoreduced EOC who undergo subsequent treatment with a combination of intravenous and intraperitoneal (IV/IP) chemotherapy (10-12). The most recent of these trials, GOG 172, demonstrated a 15.9-month improvement in OS when IP chemotherapy was used for the treatment of optimally cytoreduced, advanced EOC as compared to standard intravenous (IV) therapy (10). The results of this trial prompted an announcement by the National Cancer Institute (NCI) in 2006 recommending IP chemotherapy as the standard of care for adjuvant treatment of optimally cytoreduced, advanced EOC. However, the full benefit of IP chemotherapy for the treatment of advanced EOC has not been realized, as toxicity, IP catheter complications, and complicated dosing regimens have limited widespread adoption of this method. Variable uptake has led to interest in incorporation of hyperthermic intraperitoneal chemotherapy (HIPEC) into the treatment plan given the ease of administration at the time of surgery and the lack of an indwelling IP catheter.
Interest in HIPEC has been largely driven by success in the treatment of carcinomatosis from gastrointestinal malignancies by Sugarbaker and others (13-16). EOC exhibits a pattern of dissemination similar to that of advanced gastrointestinal malignancies in that metastases tend to develop diffusely via direct tumor spread within the peritoneal cavity as opposed to hematologic or lymphatic routes. This makes the use of HIPEC in the treatment of these patients a logical consideration. In the treatment of ovarian cancer, HIPEC has the potential benefit of overcoming some of the toxicities and challenges of administering IP chemotherapy. Hyperthermia may increase cytotoxicity of chemotherapeutic agents by increasing intracellular drug penetration and overcoming platinum resistance with hyperthermia (17). Additionally, hyperthermia itself may have a direct cytotoxic effect that acts in synergy with cytotoxic chemotherapy agents (18). Several studies have demonstrated that concentrations of chemotherapy agents within the peritoneal cavity are several times higher when chemotherapy is administered intra-abdominally than when given intravenously (19-21). IP drug delivery overcomes the peritoneal-plasma barrier and concentrates cytotoxic agents within the peritoneal cavity for prolonged periods of time which may enhance cancer cell death with the potential for less systemic toxicity.
Despite the proposed benefits of HIPEC in the treatment of EOC, the data with regard to clinical applicability remain quite heterogeneous. In a study by Deraco et al., patients with advanced primary ovarian cancer who underwent primary debulking received HIPEC with doxorubicin and cisplatin followed by standard chemotherapy. Five-year OS was 60.7% and 5-year PFS survival was 15.2% (22). In another study, Coccolini et al., reported outcomes of 54 patients with primary or recurrent EOC who underwent surgical cytoreduction followed by HIPEC with cisplatin and paclitaxel. At median follow-up of 20 months, PFS and OS were 12.5 and 32.9 months, respectively (23). Fagotti et al. reported a series of 42 patients with recurrent, platinum-sensitive EOC who were treated with cytoreduction and HIPEC with oxaliplatin followed by adjuvant IV oxaliplatin and taxotere. The toxicity profile was reasonable with a 34.8% complication rate and no perioperative deaths reported. At a median follow-up of 18 months, disease-free and OS were 24 and 38 months, respectively (24).
There is clearly a growing interest in HIPEC given the increasing number of prospective trials incorporating HIPEC; however, the ideal cytotoxic regimen and timing remains unclear. This study aims to examine patients with EOC treated with HIPEC with a particular focus on the cytotoxic regimen and treatment outcomes to guide future prospective trial design.
Materials and methods
University of Pittsburgh Institutional Review Board approval was obtained. Patients diagnosed with EOC between January 1, 2004 and December 31, 2013 who were treated with HIPEC were identified from a prospectively maintained HIPEC database. The indication for treatment and the HIPEC regimen given were left to the discretion of the treating physician. HIPEC was administered via the closed-abdomen technique as previously described in the literature (25). Patients were excluded from this study if the indication for HIPEC was a non-epithelial histology or a tumor of low malignant potential. However, patients with an initial diagnosis of a low-malignant potential tumor that recurred as an invasive malignancy that was subsequently treated with HIPEC were included.
Charts were abstracted for demographic information, disease characteristics, treatment characteristics, and patient outcomes. For each patient, available treatment details were collected for all regimens given prior to and following HIPEC administration. Indications for HIPEC treatment include front-line treatment, consolidation therapy following treatment of primary or recurrent disease, and treatment of recurrence. Unless otherwise stated, all patients underwent optimal cytoreduction prior to the administration of HIPEC. In cases where HIPEC was given as consolidative treatment after completion of therapy for primary or recurrent disease, the patient was confirmed to have no evidence of IP disease via a second-look surgery prior to administering HIPEC. Information on surgical outcomes was collected with a focus on perioperative complications and perioperative mortality. Patient outcomes were collected including time to progression following HIPEC and OS following HIPEC treatment. Toxicities were compared between treatment regimens using Fisher’s exact test with significance set at a P value of P<0.05.
Results
Thirty-four patients were included in this study. The demographic and clinicopathologic characteristics of the patients in this study are shown in Table 1. The median age of diagnosis was 56.5 years (range, 31-74 years). The majority of patients had serous tumors (28, 82%) with the remainder having clear cell (1, 3%), endometrioid (1, 3%), mucinous (3, 9%), and adenosquamous (1, 3%) histologies. At the time of presentation, the majority of patients had advanced stage disease (28, 82%). Three patients (9%) had early stage disease at presentation. In another three (9%) patients the initial disease stage was unknown because the patient either received neoadjuvant chemotherapy prior to undergoing surgery or because the patient did not undergo a complete staging procedure. One patient initially presented with a mucinous tumor of low malignant potential that recurred as a low-grade mucinous tumor that was treated with HIPEC.
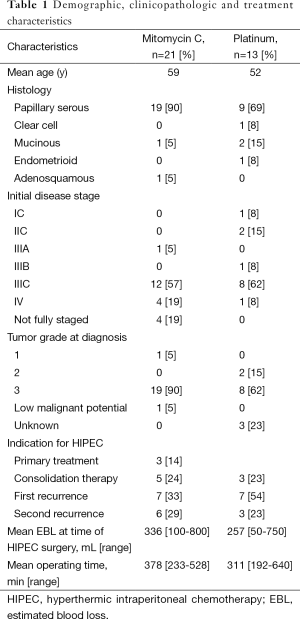
Full table
The most common indication for treatment with HIPEC was treatment of first recurrence (14, 41%). Other indications for HIPEC include primary treatment (3, 9%), treatment of second recurrence (9, 26%), consolidative treatment following treatment of primary disease (5, 15%), and consolidative treatment following treatment of disease recurrence (3, 9%). The median number of chemotherapy regimens prior to undergoing treatment with HIPEC (including front-line regimens) was two. At the time of surgical cytoreduction prior to HIPEC, 26 (76%) patients underwent optimal cytoreduction (defined as 1 cm or less of residual disease). Fifteen (44%) of patients required resection of at least one abdominal visceral organ. Mitomycin C was the most commonly used agent (21, 62%) followed by cisplatin (10, 29%), oxaliplatin (2, 6%), and carboplatin (1, 3%). Mitomycin C was most commonly given as a total dose of 40 mg while cisplatin was most commonly given at a dose of 100 mg/m2.
The median length of stay following HIPEC administration was 9 days (range, 3-39 days). It is common practice in our institution for all patients receiving HIPEC to be pre-emptively admitted to the ICU following surgery. The most common post-operative complications were hematologic with 13 (37%), 5 (14%), and 1 (3%) of patients experiencing anemia requiring blood transfusion, grade 3 or 4 neutropenia, and grade 3 or 4 thrombocytopenia, respectively (Table 2). Two (6%) patients experienced post-operative sepsis during their post-operative stay. Only two (13%) of those patients undergoing visceral resection developed an intra-abdominal abscess or fistula in the first 30 days from surgery. Seven (21%) patients developed transient renal dysfunction, defined as >50% rise in serum creatinine value over baseline as defined by the Acute Kidney Injury Criteria (26), all of which, resolved by 30 days post-operatively. One patient (3%) experienced renal dysfunction that persisted for longer than 30 days post-operatively but did not go on to require dialysis. Of the eight patients who suffered some degree of renal dysfunction, all but one had received HIPEC with a platinum agent. Of those patients who received HIPEC with cisplatin, 70% experienced some degree of renal dysfunction. When comparing toxicity profiles between mitomycin C and platinum-containing agents, only transient renal dysfunction was significantly different between the two groups (P=0.007). Of note, one patient in each the mitomycin C and platinum groups had an elevated creatinine at baseline, defined as a serum of creatinine of >1.3 mg/dL.
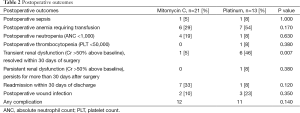
Full table
Eight (24%) patients were re-admitted within 30 days of discharge. The most common reason for re-admission was infection (six patients) with the remaining two patients being readmitted for dehydration. There were no post-operative deaths in this cohort. Following recovery from surgery, 11 (32%) patients received additional therapy. Seven of those patients received cytotoxic agents while four received treatment with tamoxifen. Two additional patients were recommended to receive additional chemotherapy, however one patient declined additional therapy and one patient was never medically well enough to receive additional therapy. At a median follow-up of 20 months (range, 3-87 months), eight patients are alive with disease, seven have no evidence of disease, 14 have died of their disease, and five patients have been lost to follow-up. Median time to progression for the group of patients receiving mitomycin C is 9 months and median time to progression for the group of patients receiving platinum is 7 months. This difference is not statistically significant (P=0.23).
Discussion
Indications for the role of HIPEC in EOC and the optimal drug regimen(s) are controversial (22-24). Zivanovic et al., in a phase I trial, demonstrated both feasibility and an acceptable toxicity profile of HIPEC utilizing cisplatin 100 mg/m2 following optimal tumor cytoreduction in the primary setting. The authors propose this dose as the recommend phase II dose in future studies (27). In our study, when comparing toxicity profiles between mitomycin C and platinum-containing agents, the rates of toxicities are similar except for the rate of transient renal dysfunction; this may be due to the difference in patient populations as the majority of our patients had at least one prior platinum based chemotherapy regimen. It is important to note that in the case of some toxicities, anemia in particular, it is difficult to determine whether the toxicity is solely a side effect of the chemotherapy agent or is related to the surgical procedure itself. Importantly, recommended adjuvant therapy was received in all but two patients and only one did not receive this therapy due to prolonged surgical recovery. This study adds to the growing body of literature demonstrating the safety and feasibility of HIPEC in the treatment of EOC. The use of platinum-based HIPEC regimens is of particular interest because most EOCs are platinum sensitive. Median time to progression is 7 months for the platinum group and 9 months for the mitomycin C group, but this difference is not statistically significant. This result is limited by the small sample size and variability in indications for HIPEC in our study.
Overall, the toxicity profile in our study is comparable to those reported in the literature. In a systemic review by Chua et al., cytoreductive surgery plus HIPEC for various indications estimated the mortality rate attributable to HIPEC therapy to be 0-5.8% with an estimated rate of major morbidities from 12-52%. The rate of hematologic morbidity in this study was 0-28% and the rate of renal failure was 0-7% (28). A recent study by Cripe et al. investigated the short-term morbidity and mortality of 32 patients undergoing HIPEC for EOC in the interval, consolidative, and recurrent setting. The majority of patients in this study received platinum-based chemotherapy. The rate of major morbidity was 65.6%, with anemia being the most common. There were no perioperative deaths in their cohort. When anemia was excluded as a complication, the rate of major morbidity was noted to be 25% (29). These studies in conjunction with previously published literature further support a reasonable side-effect profile for HIPEC in patients with EOC with an overall low risk of perioperative mortality.
A major challenge in incorporating HIPEC into the treatment for EOC is determining the most efficacious treatment regimen with the best toxicity profile. A number of different agents have been used in HIPEC protocols. Platinum-based therapy is an obvious choice because the majority of EOCs are platinum sensitive tumors. Argenta et al. published a pilot study of HIPEC with carboplatin 1,000 mg/m2 for platinum-sensitive recurrent disease which demonstrated both feasibility and safety (30). Deraco et al. conducted a phase II trial of HIPEC with cisplatin and doxorubicin in the front-line setting. The authors demonstrated a 5-year OS of 60.7% and PFS of median of 30 months or 15.2% without significant toxicity (22). This is one of the largest phase II studies exploring both efficacy and toxicity of HIPEC in EOC published to date. Oxaliplatin has also been evaluated as a potentially effective agent with a similar side effect profile to the other platinum-based regimens (24). Studies comparing carboplatin to cisplatin in EOC have demonstrated equivalence in the IV setting and there are data to suggest similar tumor penetration of both agents when administered into the peritoneal cavity (3,31). Additional prospective investigations of platinum-based regimens as HIPEC agents will help elucidate drug effectiveness, as well as better define the IP side-effect profile. Other cytotoxics that have been used in HIPEC include taxanes, doxorubicin, and mitomycin C. Both taxanes and doxorubicin are active agents in the treatment of EOC while mitomycin C derives from HIPEC regimens for gastrointestinal malignancies. At our institution, treatment with a platinum-containing agent is preferred for patients with platinum-sensitive EOC; however, it is more toxic than mitomycin C, particularly with regard to renal dysfunction. In patients who are known to be platinum-resistant or in whom performance status, medical comorbidities, or end-organ disease prohibit the use of a platinum-containing agent, mitomycin C is preferred.
Prospective data demonstrating an improvement in progression free and OS in EOC patients receiving HIPEC are lacking. Several retrospective trials have suggested a survival benefit with the addition of HIPEC to the treatment regimens of patients with recurrent EOC (32,33). Safra et al. performed a retrospective case-control study of patients treated for recurrent EOC with cytoreduction plus HIPEC versus IV chemotherapy alone. Although OS endpoints in this study have not yet been reached, the results suggest a significant improvement in PFS for the cytoreduction plus HIPEC cohort compared to chemotherapy alone (15 vs. 6 months, P=0.001) (32). In another retrospective case-controlled study by Le Brun et al., patients with first recurrence, platinum-sensitive EOC treated with either cytoreduction plus HIPEC or with a regimen that did not include HIPEC. The 4-year OS rate in the HIPEC group was 75.6% while it was only 19.4% in the non-HIPEC group (P=0.013) (33). Both studies have the benefit of having included a group of patients with a single indication for HIPEC, but, the associated selection biases cannot be completely accounted for in these retrospective studies. Unfortunately, in our study similar to much of the current literature, the patient cohort is very heterogeneous both in terms of indication for HIPEC and of treatment regimen used and thus survival endpoints could not be reported because of the small sample size. Chiva et al. estimated the weighted average of OS in trials of HIPEC following cytoreduction in the front-line and recurrent settings to be 37.6 and 36.5 months, respectively. The authors note that these results are comparable to those results noted with standard therapies and propose pursuing prospective trials of HIPEC in EOC (17).
Our study reports a series of patients treated with HIPEC for EOC at a single institution. The strength of this study is that all patients were treated with the same technique, limiting inter-institutional variability and the associated biases. Also, our study is one of the few utilizing mitomycin C in HIPEC following cytoreductive surgery for EOC patients. However, the study is limited by the inherent bias of a retrospective study with a small sample size. It is possible that mild complications of surgery may not be adequately reported in the medical record; thus, missed in this analysis. Determining the extent of cytoreduction performed retrospectively is difficult and at times not reported in the operative report. Additionally, this study lacks an assessment of patient quality of life measures, which is particularly important in patients undergoing therapy for recurrent disease. The variable indications for surgery as well as the variable chemotherapy regimens significantly impact the ability to compare outcomes data within this patient population, thus making it difficult to draw any conclusions about how HIPEC impacts outcomes for patients with EOC.
Currently, there are ongoing phases II and phase III clinical trials attempting to clarify the role of HIPEC for the treatment of EOC. Literature suggests that in appropriately selected patient populations, HIPEC may confer both a PFS and OS benefit. Our data supports a reasonable side effect profile of several HIPEC regimens for patients with EOC for multiple treatment indications. Future studies designed to determine which cytotoxic agents would provide the most benefit in patients with EOC are needed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [PubMed]
- Cannistra SA. Cancer of the ovary. N Engl J Med 2004;351:2519-29. [PubMed]
- Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 2003;21:3194-200. [PubMed]
- McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 1996;334:1-6. [PubMed]
- Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 2002;20:1248-59. [PubMed]
- Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363:943-53. [PubMed]
- Katsumata N, Yasuda M, Isonishi S, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol 2013;14:1020-6. [PubMed]
- Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol 2014;32:1302-8. [PubMed]
- Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33:244-50. [PubMed]
- Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006;354:34-43. [PubMed]
- Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 1996;335:1950-5. [PubMed]
- Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 2001;19:1001-7. [PubMed]
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737-43. [PubMed]
- Elias D, Delperro JR, Sideris L, et al. Treatment of peritoneal carcinomatosis from colorectal cancer: impact of complete cytoreductive surgery and difficulties in conducting randomized trials. Ann Surg Oncol 2004;11:518-21. [PubMed]
- Sugarbaker PH. Managing the peritoneal surface component of gastrointestinal cancer. Part 1. Patterns of dissemination and treatment options. Oncology (Williston Park) 2004;18:51-9. [PubMed]
- Sugarbaker PH. Managing the peritoneal surface component of gastrointestinal cancer. Part 2. Perioperative intraperitoneal chemotherapy. Oncology (Williston Park) 2004;18:207-19; discussion 220-2, 227-8, 230.
- Chiva LM, Gonzalez-Martin A. A critical appraisal of hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of advanced and recurrent ovarian cancer. Gynecol Oncol 2015;136:130-5. [PubMed]
- Facy O, Radais F, Ladoire S, et al. Comparison of hyperthermia and adrenaline to enhance the intratumoral accumulation of cisplatin in a murine model of peritoneal carcinomatosis. J Exp Clin Cancer Res 2011;30:4. [PubMed]
- Alberts DS, Peng YM, Chen HS, et al. Therapeutic synergism of hyperthermia-cis-platinum in a mouse tumor model. J Natl Cancer Inst 1980;65:455-61. [PubMed]
- Ansaloni L, Coccolini F, Morosi L, et al. Pharmacokinetics of concomitant cisplatin and paclitaxel administered by hyperthermic intraperitoneal chemotherapy to patients with peritoneal carcinomatosis from epithelial ovarian cancer. Br J Cancer 2015;112:306-12. [PubMed]
- de Bree E, Rosing H, Filis D, et al. Cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy with paclitaxel: a clinical and pharmacokinetic study. Ann Surg Oncol 2008;15:1183-92. [PubMed]
- Deraco M, Kusamura S, Virzì S, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy as upfront therapy for advanced epithelial ovarian cancer: multi-institutional phase-II trial. Gynecol Oncol 2011;122:215-20. [PubMed]
- Coccolini F, Campanati L, Catena F, et al. Hyperthermic intraperitoneal chemotherapy with cisplatin and paclitaxel in advanced ovarian cancer: a multicenter prospective observational study. J Gynecol Oncol 2015;26:54-61. [PubMed]
- Fagotti A, Costantini B, Vizzielli G, et al. HIPEC in recurrent ovarian cancer patients: morbidity-related treatment and long-term analysis of clinical outcome. Gynecol Oncol 2011;122:221-5. [PubMed]
- González-Moreno S, González-Bayón LA, Ortega-Pérez G. Hyperthermic intraperitoneal chemotherapy: Rationale and technique. World J Gastrointest Oncol 2010;2:68-75. [PubMed]
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [PubMed]
- Zivanovic O, Abramian A, Kullmann M, et al. HIPEC ROC I: a phase I study of cisplatin administered as hyperthermic intraoperative intraperitoneal chemoperfusion followed by postoperative intravenous platinum-based chemotherapy in patients with platinum-sensitive recurrent epithelial ovarian cancer. Int J Cancer 2015;136:699-708. [PubMed]
- Chua TC, Liauw W, Robertson G, et al. Establishing evidence for change in ovarian cancer surgery--proposing clinical trials of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer peritoneal carcinomatosis. Gynecol Oncol 2009;115:166-8. [PubMed]
- Cripe J, Tseng J, Eskander R, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for recurrent ovarian carcinoma: analysis of 30-day morbidity and mortality. Ann Surg Oncol 2015;22:655-61. [PubMed]
- Argenta PA, Sueblinvong T, Geller MA, et al. Hyperthermic intraperitoneal chemotherapy with carboplatin for optimally-cytoreduced, recurrent, platinum-sensitive ovarian carcinoma: a pilot study. Gynecol Oncol 2013;129:81-5. [PubMed]
- Jandial DD, Messer K, Farshchi-Heydari S, et al. Tumor platinum concentration following intraperitoneal administration of cisplatin versus carboplatin in an ovarian cancer model. Gynecol Oncol 2009;115:362-6. [PubMed]
- Safra T, Grisaru D, Inbar M, et al. Cytoreduction surgery with hyperthermic intraperitoneal chemotherapy in recurrent ovarian cancer improves progression-free survival, especially in BRCA-positive patients- a case-control study. J Surg Oncol 2014;110:661-5. [PubMed]
- Le Brun JF, Campion L, Berton-Rigaud D, et al. Survival benefit of hyperthermic intraperitoneal chemotherapy for recurrent ovarian cancer: a multi-institutional case control study. Ann Surg Oncol 2014;21:3621-7. [PubMed]


