Impact of surgical volume of centers on post-operative outcomes from cytoreductive surgery and hyperthermic intra-peritoneal chemoperfusion
Introduction
Cytoreductive surgery (CRS) and hyperthermic intra-peritoneal chemoperfusion (HIPEC) has evolved into an oncologically effective technique for patients with peritoneal metastases from a variety of malignancies. Akin to other complex surgical oncology operations, performance of such a procedure requires considerable expertise in the peri-operative management of the patient for both the surgeon and the peri-operative team. In this article, we explore the premise of higher surgical volume improving peri-operative outcomes for patients undergoing CRS + HIPEC as well as its ramifications.
Problem statement
CRS and HIPEC is currently applied to patients with peritoneal dissemination from appendiceal cancer, mesothelioma, colorectal cancer, gastric cancer and ovarian cancer commonly and to desmoplastic small round cell tumors and sarcomas infrequently. Given the diverse histology groups included in the target population, it is difficult to estimate the true burden of patients who might benefit from this therapy. However, conservative estimates based on published data suggest that the annual burden of patients in the United States eligible for consideration of CRS + HIPEC is 29,260-40,890 (Table S1).

Full table
Estimating the true incidence of patients undergoing CRS and HIPEC in the United States is difficult. There is no current procedural terminology (CPT) code that encompasses the cytoreduction, the instillation of intra-peritoneal chemotherapy and the generation of hyperthermia. Additionally, varied ICD-10 procedure and diagnosis codes utilized in the practice make it difficult to ascertain such information from administrative/claims databases. Furthermore, registries such as the Surveillance End Results and Epidemiology Registry (SEER) or National Cancer Data Base (NCDB) do not capture this information separately.
The National Surgical Quality Improvement Project collects data on 653 hospitals of the 5,686 (11%) hospitals in the United States (1,2). CPT code combinations have been used to ascertain the number of patients that underwent intra-peritoneal chemotherapy concurrent with cytoreduction in these hospitals and includes 795 patients from 2005-2011 (6 years; estimated 132 patients/year) and 694 patients from a separate report in the same time period (3,4). Assuming uniform population distribution in the hospitals is fallacious but would yield an estimate of 1,521 patients per year.
Upon examining the volumes of centers reporting outcomes on more than 500 patients, it is apparent that the number of HIPEC procedures performed over a long time period yields a simple rate of 50 cases/year assuming no growth and 55 per year assuming annual growth rate of 3% (Table 1). While such estimates are likely inaccurate, it is important to understand the scale of the problem before understanding the impact of surgical volume on outcomes.
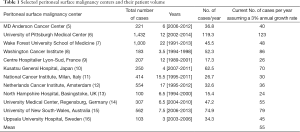
Full table
Center volume and outcomes in oncological surgery
It has been suggested that hospital volume is a proxy measure of superior outcomes in numerous oncological operations (17,18). The association between increasing hospital volume and better peri-operative outcomes appears to be more clearly seen with increasing complexity of the operations. Large differences in mortality and morbidity have been seen with oncological operations such as pancreatectomies, esophagectomies, colectomies, pancreatoduodenectomies and gastrectomies which are commonly performed during CRS (19). The volume of a center may have a causal effect on the improved outcomes, although likely the volume is an indicator of other processes that improve outcomes. While the volume-outcome relationship has not been studied in centers performing CRS + HIPEC, factors associated with alteration of outcome in higher volume centers are shown in Table 2. The majority of the studies examining volume-outcome data use administrative/claims data to support their hypothesis (20). Specific registry data such as the VA-NSQIP, which includes detailed peri-operative data collection in a standardized fashion, has, however, not shown the same strength of association between volume of a center and outcome (21,22).

Full table
While it is assumed that higher hospital volume leads to higher per surgeon cases, this inference is not always true. Some studies have suggested that regardless of surgeon volume, increased hospital volume can reduce complications (23). Yet the most appealing and clinically intuitive argument asserts that gaining proficiency in both operating and systems of care has the most significant impact on the outcomes for patients. A systematic review in 2007 examined over 127 studies for hospital volume and 58 studies which included surgeon volume and concluded that high volume surgeons and specialists had significantly reduced complications, although the hospital volume did not play as important a role in the outcomes (19). Such a systematic review has not been repeated in the past 5 years.
Learning curve for CRS + HIPEC
Numerous studies over the years have examined the effect of the learning curve. It is clear that technical proficiency and improvement of systems of care occur over time and with repetition. In addition, patient selection and prediction of morbidity also improves over time. While the former is represented in being more selective and conservative in operating on patients, gain of technical proficiency and prediction of morbidity is represented by occasionally operating on more challenging, complex cases. For purposes of examining this, we have divided the studies into two groups—those that include cohorts separated by temporality in the performance of the procedures and those that have used risk adjusted probability models to detect the “inflection point” of the curve.
Consecutive cohort studies
Scientific groups from UK, Netherlands and Australia examined their cumulative experience to discern the effect of learning on performance of CRS. Moran divided their cohort into three consecutive groups of 33 patients each (one group had 34) and found that over time, there was improved patient selection (fewer patients underwent surgery 61% reduced to 37%) and reduced morbidity and mortality (18% mortality reduced to 3%, 27% morbidity reduced to 0%) (13). The Dutch group similarly examined 323 procedures performed over 10 years. The cohorts were divided by time periods and separated by histology. The simplified PCI score reduced over time for both peritoneal carcinomatosis (PC) and appendiceal histologies, while the R1 cytoreduction rate increased over time (47% to 74% PC, 15% to 49% appendiceal) (24). The Australian group similarly examined their first 70 patients with the subsequent 70 patients and found that while they operated on patients with more disease (PCI ≥20 in 37.1% compared to 18.6% previously), the completeness of cytoreduction score remained the same. In addition, the severe morbidity rates decreased from 30% to 10% in this time period (25).
Adjusted models
Three adjusted models have examined the learning curve for CRS + HIPEC. Andreasson et al. used the partial least squares (PLS) method and the cumulative sum control chart (CUSUM) to examine the learning curve. In the cohort of 128 patients, stabilization of the curve was seen after 220 procedures, and comparing the first 73 patients to the subsequent 55 patients revealed better patient selection (65% low grade histology vs. 34% previously) with similar burden of disease reflected by PCI scores (26). The completeness of cytoreduction (48% vs. 80% R1) and the overall survival were significantly improved in the latter cohort. Kusamura et al. and Polanco et al. used their prospective cohorts from large institutions to create a risk adjusted sequential probability ratio test (11,27). This plot compared the composite outcome of suboptimal cytoreduction and grade 3-5 morbidity to a pre-specified odds ratio and error rate. Consecutive hypothesis testing occurred and risk was predicted by using logistic regression models which allowed for risk adjusted cohorts. This method is superior in discerning the surgical aggressiveness that occurs with technical proficiency with the superior selection and improvement of systems of care. Both studies found an inflection point of 140 and 180 patients respectively before technical proficiency occurred (11). Oncological proficiency was calculated in the study by Polanco and was achieved at 90 patients (Table 3) (27).
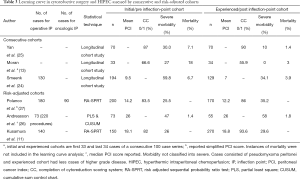
Full table
Implications of learning curve for regionalization of care
Most studies that have examined an inflection point in technical expertise found that around 140-220 cases need to be performed before such expertise is reached. If a new center were to aim to achieve expertise within 5 years, this would require the center to perform 28-44 HIPEC procedures per year. Considering data for individual surgeons such as reports from UK or Australia, it appears that the learning curve can be achieved with 33-70 cases for an individual surgeon. Currently in the United States, such annual volumes are encountered only at a few major regional centers. The argument for regionalization of care is robust; it leads to more proficient teams, surgeons and better systems of care. However, regionalization of care comes with difficulties in travel especially for elderly patients and disenfranchises providers in non-referral hospitals. Further, it mitigates the ability to truly study a system of care and attempt to improve it. In addition, transition of population to urban referral facilities might overload them and thus compromise care. Conversely, performing infrequent procedures with an ill-equipped team and without studying outcomes is certainly a disservice to our patients.
Upon examining the initial learning curve of centers that embraced this procedure early, it is apparent that this procedure occurred with significant morbidity and mortality. We compared this, however, to reports from recently initiated centers in different parts of the world, specifically with expertise help from established centers (28). It is apparent from these early reports from the newly established centers in the United States, Italy, Germany, Colombia and Mexico that the learning curve can clearly be shortened with training, expertise, and mentorship (Table 4) (29,30,32,33). Yet, it is distinctly possible that this could be a reflection of publication bias, where by only centers with positive outcomes report them in the literature. Uniform reporting mechanisms are essential to ensure that all centers, whether high or low volume, are measured for their risk adjusted performance against their peers to improve performance. Some of the suggested strategies in reducing time to proficiency are outlined in Table 5.

Full table

Full table
Conclusions
In summary, development of surgical, technical and oncological proficiency occurs with accruing experience that requires center volume. Efforts to improve delivery of care in the United States must focus on improving surgical proficiency, improving systems of care and create a reporting mechanism to study outcomes.
Acknowledgements
Sarah Chrabaszcz assisted in reviewing the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American College of Surgeons National Surgical Quality Improvement Program® (ACS NSQIP®). Available online: https://www.facs.org/quality-programs/acs-nsqip
- American Hospital Association. Fast Facts on US Hospitals. Available online: http://www.aha.org/research/rc/stat-studies/fast-facts1.shtml
- Jafari MD, Halabi WJ, Stamos MJ, et al. Surgical outcomes of hyperthermic intraperitoneal chemotherapy: analysis of the american college of surgeons national surgical quality improvement program. JAMA Surg 2014;149:170-5. [PubMed]
- Bartlett EK, Meise C, Roses RE, et al. Morbidity and mortality of cytoreduction with intraperitoneal chemotherapy: outcomes from the ACS NSQIP database. Ann Surg Oncol 2014;21:1494-500. [PubMed]
- Owusu-Agyemang P, Soliz J, Hayes-Jordan A, et al. Safety of epidural analgesia in the perioperative care of patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2014;21:1487-93. [PubMed]
- UPMC. Hyperthermic Intraperitoneal Chemoperfusion (HIPEC) Treatment. Available online: http://www.upmc.com/Services/regional-perfusion/treatment/hipec/Pages/default.aspx
- Levine EA, Stewart JH 4th, Shen P, et al. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg 2014;218:573-85. [PubMed]
- Stephens AD, Alderman R, Chang D, et al. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol 1999;6:790-6. [PubMed]
- Glehen O, Osinsky D, Cotte E, et al. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol 2003;10:863-9. [PubMed]
- Mizumoto A, Canbay E, Hirano M, et al. Morbidity and mortality outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy at a single institution in Japan. Gastroenterol Res Pract 2012;2012:836425.
- Kusamura S, Baratti D, Deraco M. Multidimensional analysis of the learning curve for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal surface malignancies. Ann Surg 2012;255:348-56. [PubMed]
- Kuijpers AM, Mirck B, Aalbers AG, et al. Cytoreduction and HIPEC in the Netherlands: nationwide long-term outcome following the Dutch protocol. Ann Surg Oncol 2013;20:4224-30. [PubMed]
- Moran BJ. Decision-making and technical factors account for the learning curve in complex surgery. J Public Health (Oxf) 2006;28:375-8. [PubMed]
- Glockzin G, von Breitenbuch P, Schlitt HJ, et al. Treatment-related morbidity and toxicity of CRS and oxaliplatin-based HIPEC compared to a mitomycin and doxorubicin-based HIPEC protocol in patients with peritoneal carcinomatosis: a matched-pair analysis. J Surg Oncol 2013;107:574-8. [PubMed]
- Vukadinovic V, Chiou JD, Morris DL. Clinical features of pulmonary emboli in patients following cytoreductive surgery (peritonectomy) and hyperthermic intraperitoneal chemotherapy (hipec), a single centre experience. Eur J Surg Oncol 2015;41:702-6. [PubMed]
- van Leeuwen BL, Graf W, Pahlman L, et al. Swedish experience with peritonectomy and HIPEC. HIPEC in peritoneal carcinomatosis. Ann Surg Oncol 2008;15:745-53. [PubMed]
- Harmon JW, Tang DG, Gordon TA, et al. Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg 1999;230:404-11; discussion 411-3. [PubMed]
- Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998;280:1747-51. [PubMed]
- Chowdhury MM, Dagash H, Pierro A. A systematic review of the impact of volume of surgery and specialization on patient outcome. Br J Surg 2007;94:145-61. [PubMed]
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128-37. [PubMed]
- Khuri SF, Henderson WG. The case against volume as a measure of quality of surgical care. World J Surg 2005;29:1222-9. [PubMed]
- Khuri SF, Daley J, Henderson W, et al. Relation of surgical volume to outcome in eight common operations: results from the VA National Surgical Quality Improvement Program. Ann Surg 1999;230:414-29; discussion 429-32. [PubMed]
- Urbach DR, Baxter NN. Does it matter what a hospital is "high volume" for? Specificity of hospital volume-outcome associations for surgical procedures: analysis of administrative data. BMJ 2004;328:737-40. [PubMed]
- Smeenk RM, Verwaal VJ, Zoetmulder FA. Learning curve of combined modality treatment in peritoneal surface disease. Br J Surg 2007;94:1408-14. [PubMed]
- Yan TD, Links M, Fransi S, et al. Learning curve for cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal surface malignancy--a journey to becoming a Nationally Funded Peritonectomy Center. Ann Surg Oncol 2007;14:2270-80. [PubMed]
- Andréasson H, Lorant T, Påhlman L, et al. Cytoreductive surgery plus perioperative intraperitoneal chemotherapy in pseudomyxoma peritonei: aspects of the learning curve. Eur J Surg Oncol 2014;40:930-6. [PubMed]
- Polanco PM, Ding Y, Knox JM, et al. Institutional learning curve of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for peritoneal malignancies. Ann Surg Oncol 2015;22:1673-9. [PubMed]
- Kusamura S, Baratti D, Virzì S, et al. Learning curve for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal surface malignancies: analysis of two centres. J Surg Oncol 2013;107:312-9. [PubMed]
- Tabrizian P, Shrager B, Jibara G, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis: outcomes from a single tertiary institution. J Gastrointest Surg 2014;18:1024-31. [PubMed]
- Kerscher AG, Mallalieu J, Pitroff A, et al. Morbidity and mortality of 109 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC) performed at a community hospital. World J Surg 2010;34:62-9. [PubMed]
- Konstantinidis IT, Young C, Tsikitis VL, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion: The University of Arizona early experience. World J Gastrointest Surg 2012;4:135-40. [PubMed]
- Arias F, Herrera-Almario G, Pozo ME, et al. Safety and quality outcomes in peritoneal surface malignancy patients: developing a national center for excellence in Colombia. Ann Surg Oncol 2015;22:1733-8. [PubMed]
- García-Matus R, Hernández-Hernández CA, Leyva-García O, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal carcinomatosis: initial experience in Oaxaca, Mexico. Am Surg 2012;78:942-6. [PubMed]
- Turrini O, Lambaudie E, Faucher M, et al. Initial experience with hyperthermic intraperitoneal chemotherapy. Arch Surg 2012;147:919-23. [PubMed]
- Gusani NJ, Cho SW, Colovos C, et al. Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann Surg Oncol 2008;15:754-63. [PubMed]
- Kuijpers AM, Aalbers AG, Nienhuijs SW, et al. Implementation of a standardized HIPEC protocol improves outcome for peritoneal malignancy. World J Surg 2015;39:453-60. [PubMed]
- Levine EA, Stewart JH 4th, Russell GB, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg 2007;204:943-53; discussion 953-5. [PubMed]
- Schmidt U, Dahlke MH, Klempnauer J, et al. Perioperative morbidity and quality of life in long-term survivors following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol 2005;31:53-8. [PubMed]
- Surveillance, Epidemiology, and End Results Program. Cancer Stat Fact Sheets. Available online: http://seer.cancer.gov/statfacts/more.html
- Franko J, Shi Q, Goldman CD, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol 2012;30:263-7. [PubMed]
- Jayne DG, Fook S, Loi C, et al. Peritoneal carcinomatosis from colorectal cancer. Br J Surg 2002;89:1545-50. [PubMed]
- American Cancer Society. Cancer Facts & Figures 2015. Available online: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf
- Henley SJ, Larson TC, Wu M, et al. Mesothelioma incidence in 50 states and the District of Columbia, United States, 2003-2008. Int J Occup Environ Health 2013;19:1-10. [PubMed]


