Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for gastric cancer and other less common disease histologies: is it time?
Introduction
Gastric cancer, the fourth most common newly diagnosed cancer worldwide, carries an incontrovertible mortality burden with a five-year survival rate of ~25% for all stages (1,2). Up to 40% of gastric cancer patients develop some type of peritoneal spread during the course of their disease, after which their 5-year survival drops to less than 5% (3-5). Those afflicted by peritoneal carcinomatosis from gastric cancer are currently treated as stage IV, receiving systemic chemotherapies with generally bleak results. Indeed, only a minority of patients survive longer than one year and nearly all present challenges to palliation, frequently exacerbated due to common GI failure, in the final weeks of life (6).
The need for therapies addressing peritoneal carcinomatosis in gastric cancer, combined with an emergence of cytoreductive surgery (CRS) and heated intraperitoneal chemotherapy (HIPEC) in other GI cancers, has led to a number of clinical trials seeking to establish a role for this modality in gastric cancer. This regionally focused approach is built on the concept of maximizing drug delivery to the afflicted surfaces while simultaneously elongating the therapeutic window by reducing systemic toxicity. Indeed, in a large phase III clinical trial in colorectal cancer spread to the peritoneum, HIPEC and CRS extended median survival from 12.6 to 22.3 months (P=0.032) (7). Likewise, small trials and a meta-analysis have indicated an association with prolonged survival when applying this technique to stage IV gastric cancer with peritoneal carcinomatosis (8-10). High procedure-related morbidity and mortality associated with the CRS-HIPEC approach, however, have sparked a debate on its merit. With the advent of regulatory approval of more effective as well as novel, more personalized treatment options in stage IV gastric cancer, along with advances in tailoring investigational agents specifically for peritoneal delivery, there clearly is a need to outline the appropriate role of CRS-HIPEC in this disease (1,11,12).
The primary rational for a regional perfusion approach is the ability to target the tumor burden with up to 20-times higher concentrations of drug measured in the intraperitoneal compartment compared to plasma drug level (13,14). The issue of drug penetration and delivery is particularly important in the diffuse form of gastric cancer, which, together with pancreatic adenocarcinoma, is a prime example of a malignancy with a desmoplastic inflammatory stroma, high interstitial pressures and poor vascularization (15,16). On one hand, pharmacological manipulation has been shown to exploit a tumor’s natural enhanced permeability and retention effect (EPR) by increasing leakage, extravasation, and retention of drug in the tumor tissue via greater permeability due to reduced fibrosis and interstitial pressure (16). On the other hand, direct exposure of tumor deposits to chemotherapy is thought to penetrate superficial cell layers only, and the effect of intraperitoneal chemotherapy may be mediated through rapid systemic absorption and recirculation, potentially achieving higher intratumoral concentrations than direct drug penetration (16-18).
Additionally, the evolving understanding of the heterogenetic landscape of cancer may soon require an approach individualized to metastatic site. Whole genome sequencing (WGS) studies in pancreas and renal cell cancer for example, have sampled multiple metastatic sites and elicited considerable genetic heterogeneity in both somatic mutations as well as chromosomal structural variants at different organ sites within individual patients (19,20). Further recent work has used WGS to identify patients that will have a robust or complete response to platinum-based chemotherapy (21), and it is conceivable that the choice of regional chemotherapy should be guided in the future by unique genotypic signatures of metastatic sites to optimize drug selection. Hence, the merit of individualization based on both histopathology and genotype in the selection of regional drug approaches might be particularly important in metastatic gastric cancer involving the peritoneal surface. Figure 1 shows an example of the diffuse form of gastric cancer, which is more commonly associated with peritoneal spread than the intestinal subtype of gastric adenocarcinoma. Figure 2 shows the considerable variability in cytoarchitecture, tumor cellularity, stromal expansion, and E-cadherin expression across a number of peritoneal surface lesions removed from different patients during CRS.
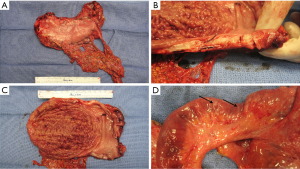
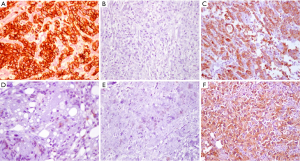
Hence, it is unlikely that a ‘one size fits all’ is the most effective approach, and the choice of chemotherapeutic regimens, including intraperitoneal therapy for peritoneal involvement, may soon depend on the genetic make-up of both primary and metastatic lesions. Here, we review the currently available data on the use of CRS in combination with HIPEC in gastric cancer, efforts to select patients and reduce morbidity of these procedures, as well as highlight advances of regional chemotherapy approaches in less common histologies, such as adrenocortical cancer (ACC) and abdominal sarcomatosis.
Retrospective evaluations of cytoreductive surgery (CRS) and HIPEC versus systemic chemotherapy for peritoneal carcinomatosis from gastric cancer
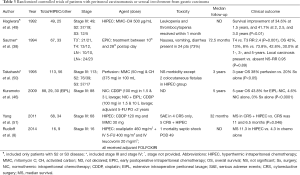
Given the rarity and frailty of patients with gastric cancer metastatic to the peritoneum, it is inherently difficult to study such cases clinically. It is thus important to first demonstrate that a new treatment modality can achieve outcomes superior to historical controls receiving standard of care. Indeed, in other GI cancers, retrospective experiences that have to date not been subjected to randomized controlled have led to accepted standards in treatment. Such was the case with the introduction of surgery for the management of colorectal liver metastases in the 1990s, as well as the use of CRS and HIPEC in the management of apppendiceal carcinoma or peritoneal mesothelioma through the pivotal work of Dr. Sugarbaker (23). Accordingly, there are a number of well-conducted retrospective series from high-volume peritoneal surface malignancy centers reporting on outcome of patients with gastric cancer with peritoneal carcinomatosis being treated with CRS and HIPEC. Table 1 details these reports including number of patients per study, median follow-up, regional chemotherapy used, treatment related complications, and clinical outcome.
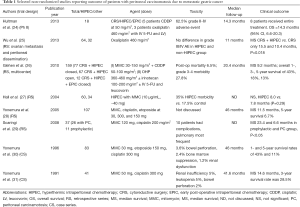
Full table
Two studies deserve to be highlighted: in the largest study with the most comprehensive follow-up French investigators describe a multi-institutional series of 159 patients treated with CRS and HIPEC and reported 1-, 2-, and 5-year survival rates of 43%, 18%, and 13%, respectively (26). Also, the study by Hall et al. from a high-volume peritoneal surface malignancy center is remarkable as it reported equal 1- and 2-year outcomes between patients with peritoneal carcinomatosis that underwent resection with complete CRS followed by HIPEC and patients who underwent radical gastrectomy without peritoneal involvement (27). Both studies reported that outcome was most favorable when a complete surgical cytoreduction could be accomplished.
For the majority of patients listed in Table 1, patients had already received at least one line of systemic chemotherapy. The observed results are thus in contrast to those in which the majority of patients treated with systemic chemotherapy only succumb to their disease within the first year. Data from Memorial Sloan Kettering Cancer Center, for example, has shown a median survival of less than 12 months for metastatic gastric cancer treated with chemotherapy only. Furthermore, metastatic disease evidenced by cytology only was not associated with improved survival (32). Other investigators have shown a similarly significant detriment to survival conferred by isolated positive peritoneal cytology (33). Subsequent work from the Memorial group, however, suggests that a multimodality approach of neoadjuvant systemic chemotherapy and surgical resection in patients with M1Cyt+ disease that reverts to negative cytology might be associated with improved disease specific survival (34). Efforts to sterilize the peritoneal compartment in combination with curative resection have been tested in a multicenter randomized trial, which implemented intraperitoneal chemotherapy along with high volume peritoneal lavage in 88 M1Cyt+ patients and is discussed in Table 2 and the next section “HIPEC as an adjuvant treatment for patients with resectable gastric cancer” (45).
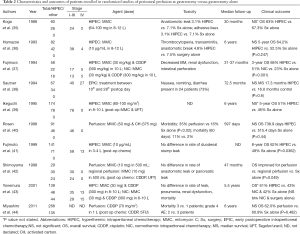
Full table
Overall, acknowledging the shortcomings of retrospective series with their inherent selection bias, the data suggests a subset of patients treated with the multimodality approach of CRS combined with HIPEC whose outcome is different from that expected for stage IV patients treated with systemic chemotherapy only.
HIPEC as an adjuvant treatment for patients with resectable gastric cancer
CRS and perfusion of the peritoneal compartment with heated chemotherapy as part of a multimodality approach are likely synergistic therapies. It is well established that smaller tumor burdens aid the efficacy of a sterilizing cytotoxic chemotherapy—a guiding principle of adjuvant chemotherapy (46). Indeed, several studies in Table 1 support the observation that complete cytoreduction prior to HIPEC is associated with improved survival. Further, several phase II studies looking at HIPEC administered at time of potentially curative resections for gastric cancer have indicated that regional chemotherapy carries therapeutic activity. Table 2 lists characteristics and outcomes of patients, without preoperatively confirmed peritoneal disease, that were randomized to peritoneal perfusion at time of gastrectomy (either as hyper- or normothermic regional chemotherapy; and in one series as early post-operative perfusion) versus gastrectomy alone.
In summary, despite the inclusion of some stage IV patients that had peritoneal involvement, the majority of these studies demonstrate improved outcomes including overall survival in patients receiving intraoperative peritoneal chemotherapy. When analyzed in a recent meta-analysis, even patients with limited peritoneal carcinomatosis that randomized to CRS and HIPEC seemed to fare better than those that received curative gastrectomy only (47). The most common morbidity of the addition of peritoneal regional chemotherapy included neutropenia and thrombocytopenia. There were no associated mortalities. This data supports an emerging role for intraoperative peritoneal chemotherapy in gastric cancer, including in patients with both a low and high risk for future peritoneal involvement as well as a limited peritoneal surface disease burden.
Cytoreductive surgery (CRS) and HIPEC in patients with known peritoneal carcinomatosis from gastric cancer
There are now promising results from long term follow-up studies on the outcomes of CRS and HIPEC in patients with peritoneal carcinomatosis from colorectal cancer available (48). These data show improved outcomes in patients treated with the multimodality approach together with the studies on the use of intraperitoneal chemotherapy in the adjuvant setting, provide a solid rationale for a prospective randomized evaluation of CRS and HIPEC for gastric cancer. Table 3 summarizes clinical studies which randomized gastric cancer patients with stage IV disease to CRS and HIPEC (or early post-operative perfusion) versus standard of care.
Full table
Some of these studies, while initially designed to evaluate HIPEC in the adjuvant setting in patients who could undergo a potentially curative resection, include separate analyses of patients that were unexpectedly found to be stage IV at operation but still underwent resection of serosal deposits followed by HIPEC. Some of these stage IV patients only had positive cytology (M1Cyt+). Both 1- and 2-year mortality rates were superior in those who received intraoperative intraperitoneal chemotherapy while 5-year mortality rates did not differ between the groups (47). These findings are affirmed by the results of the so far largest randomized clinical trial on the subject: Yang and coworkers randomized 68 patients with peritoneal carcinomatosis due to gastric cancer to either CRS alone versus CRS plus HIPEC and showed a small but statistically significant survival improvement in those with peritoneal involvement that received both CRS and HIPEC (51). The design of Yang’s and co-workers study was different from the recently presented GYMSSA study where patients were randomized to gastrectomy, CRS, and HIPEC followed by 2nd line FOLFOXFIRI versus FOLFOXIRI chemotherapy alone (8). These patients had all undergone diagnostic laparoscopy before randomization to assess peritoneal disease burden. While this study did not meet its accrual target and thus remains underpowered, the findings of several patients living beyond one year (one beyond 4 years) in the multimodality arm compared to all patients dying of their disease within one year in the chemotherapy only arm is noteworthy.
Complications associated with CRS and HIPEC in gastric cancer patients
While the above early, albeit immature, data might point to an emerging role of this approach in the management of metastatic gastric cancer with peritoneal involvement, a concept of clinical equipoise between potentially promising findings and the related risks and burden of the procedure—which are substantial—should be applied. It should also be noted that there is likely a publication bias leading to underreporting of negative findings, however, these reports do exist (52). The main toxicities reported from this approach are neutropenia, particularly in the early post-operative period, as well as GI toxicity, including leaks and fistulas. A number of studies suggest surgical techniques to reduce the likelihood of GI complications. These include (I) complete drainage of the peritoneal chemotherapy effluent followed by extensive washing prior to reestablishing GI continuity or closure; (II) the re-resection of intestinal ends (up to 1 cm) prior to anastomosis in order to join fresh ends which were not exposed to the regional chemotherapy; or (III) avoidance of excessive peritoneal stripping (53).
Despite a relatively common surgical approach there was considerable heterogeneity in the toxicities in these studies. Some reported hardly any leaks or no severe GI toxicity while others, such as the GYMSSA trial, had a high (≥20 percent) 90-day mortality rate with a limited number of patients receiving the planned adjuvant FOLFOXIRI chemotherapy. The reason for toxicity variation is unknown; potential causes include higher peritoneal disease burden, greater proportion of total gastrectomies compared to partial gastrectomies, or the administration of another 2nd line adjuvant chemotherapy regimen (FOLFOXIRI). There was no detectable correlation identifiable between the type of intraperitoneal chemotherapy administered and post-procedure complications. All studies do recommend for these procedures to be performed at high volume peritoneal surface malignancy centers.
Cytoreductive surgery (CRS) and HIPEC in less common diseases
CRS with HIPEC has been associated with improved outcomes for peritoneal carcinomatosis caused by various histologies such as peritoneal mesothelioma, appendiceal, ovarian, and colorectal cancer (48,51,54-56). However, there are still other histologies, such as those cancers that tend to have confined peritoneal disease without signs of systemic metastasis, which may benefit from HIPEC and warrant further study.
CRS and HIPEC in abdominal sarcomatosis
One such example is abdominal soft tissue sarcoma, which tends to present with early peritoneal recurrence and no distant metastasis (57). These patients have a median survival of 13 months and both surgical resection and chemotherapy have failed to show durable responses (58). In a study by Hunt et al., 28 patients underwent CRS and HIPEC over a 5-year period with either cisplatin or a cisplatin/mitoxantrone combination in two separate phase I trials (59). In patients that received HIPEC with cisplatin, the median survival was 16.9 months, while patients who received HIPEC with the combination treatment had a median survival of 5.5 months only. Complication rates were significant, 60% of the cisplatin group and 90% of the combination group developed grade 3/4 toxicities. Another study by Choudry et al. examined CRS and HIPEC in 15 patients with recurrent sarcomatosis of varying histologies (60). After CRS and chemoperfusion with mitomycin, cisplatin or doxorubicin, overall survival was 22.6 months. Grade 3/4 complications occurred in 24% of the patients. There has also been interest in exploring the role of CRS and HIPEC in a specific type of abdominal sarcoma, gastrointestinal stromal tumors. Bryan et al. retrospectively reviewed 16 patients that received CRS/HIPEC for GIST-induced sarcomatosis and found a median overall survival of 3.33 years (61). The authors, and others, speculate that debulking followed by first- and second-line tyrosine kinase inhibitor therapy in the form of imatinib (Gleevec®) or sunitinib (Sutent®) reduces, or delays, the risk of relapse due to the delayed formation of resistant clones in the tumor.
Taken together, these results might support the use of the multimodality CRS and HIPEC approach in some patients afflicted by abdominal sarcomatosis, however, toxicity can be substantial and indicates a need for diligent patient selection in future clinical trials. Critically, a randomized study with a non-HIPEC control arm has not yet been performed and additional trials are warranted.
CRS and HIPEC in adrenocortical cancer (ACC)
ACC is a rare tumor with a poor prognosis. Mortality is in the 75-90% range over 5 years, and average survival from time of diagnosis is 14.5 months with 60% of patients eventually developing with unresectable intrabdominal disease (62,63). Systemic therapy for these patients is associated with a poor response and no effect on overall survival (64,65). Indeed, the largest randomized trial for metastatic ACC with etoposide, doxorubicin, and cisplatin showed a progression free survival of 5 months and a 23% response rate (66). Considering the lack of effective systemic treatments for patients with metastatic disease, more effective therapies are needed.
At the National Cancer Institute (NCI), a retrospective analysis was performed of 14 patients with peritoneal recurrence from ACC who were treated with post-operative EDP chemotherapy. Patients who did and did not respond to chemotherapy had an average survival of 30 and 14 months, respectively. This suggests that response to chemotherapy may correlate with an increase in survival. Furthermore, considering the advantages of the regional chemotherapy approach it gives reason to believe that there may be added benefit for these patients that respond, when HIPEC is applied directly to the tumor bed. Given these findings, a trial is currently being conducted at the NCI to establish the efficacy of CRS and HIPEC for peritoneal recurrence of ACC (67). Given the efficacy of systemic cisplatin in ACC, as well as the higher tolerated dosing when given as a heated perfusate, the investigators of the trial hypothesize that patients will achieve prolonged disease free and overall survival.
Conclusions
Currently, there is still limited available data and literature defining a role for CRS and HIPEC in the management of patients with advanced gastric cancer, and further clinical research on this approach is still needed. Results thus far have suggested that CRS and HIPEC may have a role in select patients; those with a low peritoneal disease burden that can be completely reduced, or with disease that is positive by cytology only, are likely the best candidates for the approach. Clinical decisions should be made with the knowledge that toxicities can be substantial, and it is unlikely a curative option. Studies on CRS and HIPEC applied to less common diseases like soft tissue sarcomas or ACC metastatic to the peritoneal surface, while hampered by an inherent heterogeneity of included patients and histologies, mirror the trend observed in management of metastatic gastric cancer experiences but remain too scarce to give any general recommendations. Further development will require the establishment of a robust clinical trial framework at cooperating centers of excellence and more meaningful improvement in outcome will likely require the addition of novel drugs, or drug combinations, taking the unique site-specific genotype of metastases to different organs and compartments into account.
Acknowledgements
The authors would like to thank Marybeth Hughes, MD, and Tito Fojo, MD, PhD, for sharing their unpublished experience with adrenocortical cancer.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Berretta M, Fisichella R, Borsatti E, et al. Feasibility of intraperitoneal Trastuzumab treatment in a patient with peritoneal carcinomatosis from gastric cancer. Eur Rev Med Pharmacol Sci 2014;18:689-92. [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Sarela AI, Miner TJ, Karpeh MS, et al. Clinical outcomes with laparoscopic stage M1, unresected gastric adenocarcinoma. Ann Surg 2006;243:189-95. [PubMed]
- Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol 2009;472:467-77. [PubMed]
- Cappellani A, Zanghi A, Di Vita M, et al. Clinical and biological markers in gastric cancer: update and perspectives. Front Biosci (Schol Ed) 2010;2:403-12. [PubMed]
- Bonenkamp JJ, Songun I, Hermans J, et al. Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. Br J Surg 1996;83:672-4. [PubMed]
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737-43. [PubMed]
- Rudloff U, Langan RC, Mullinax JE, et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol 2014;110:275-84. [PubMed]
- Huang CQ, Feng JP, Yang XJ, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from colorectal cancer: a case-control study from a Chinese center. J Surg Oncol 2014;109:730-9. [PubMed]
- Yan TD, Black D, Sugarbaker PH, et al. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol 2007;14:2702-13. [PubMed]
- Gunn AJ, Brechbiel MW, Choyke PL. The emerging role of molecular imaging and targeted therapeutics in peritoneal carcinomatosis. Expert Opin Drug Deliv 2007;4:389-402. [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [PubMed]
- Howell SB, Pfeifle CE, Wung WE, et al. Intraperitoneal cis-diamminedichloroplatinum with systemic thiosulfate protection. Cancer Res 1983;43:1426-31. [PubMed]
- Pretorius RG, Hacker NF, Berek JS, et al. Pharmacokinetics of Ip cisplatin in refractory ovarian carcinoma. Cancer Treat Rep 1983;67:1085-92. [PubMed]
- Zhang M, Zhu G, Zhang H, et al. Clinicopathologic features of gastric carcinoma with signet ring cell histology. J Gastrointest Surg 2010;14:601-6. [PubMed]
- Kano MR, Bae Y, Iwata C, et al. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-beta signaling. Proc Natl Acad Sci U S A 2007;104:3460-5. [PubMed]
- Komuro A, Yashiro M, Iwata C, et al. Diffuse-type gastric carcinoma: progression, angiogenesis, and transforming growth factor beta signaling. J Natl Cancer Inst 2009;101:592-604. [PubMed]
- Ward BG, Shepherd JH, Monaghan JM. Occult advanced cervical cancer. Br Med J (Clin Res Ed) 1985;290:1301-2. [PubMed]
- Campbell PJ, Yachida S, Mudie LJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 2010;467:1109-13. [PubMed]
- Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467:1114-7. [PubMed]
- Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518:495-501. [PubMed]
- Beane JD, Griffin KF, Levy EB, et al. Duodenal ischemia and upper GI bleeding are dose-limiting toxicities of 24-h continuous intra-arterial pancreatic perfusion of gemcitabine following vascular isolation of the pancreatic head: early results from the Regional Chemotherapy in Locally Advanced Pancreatic Cancer (RECLAP) study. Invest New Drugs 2015;33:109-18. [PubMed]
- Sugarbaker PH. Review of a personal experience in the management of carcinomatosis and sarcomatosis. Jpn J Clin Oncol 2001;31:573-83. [PubMed]
- Hultman B, Lind P, Glimelius B, et al. Phase II study of patients with peritoneal carcinomatosis from gastric cancer treated with preoperative systemic chemotherapy followed by peritonectomy and intraperitoneal chemotherapy. Acta Oncol 2013;52:824-30. [PubMed]
- Wu XJ, Yuan P, Li ZY, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves the survival of gastric cancer patients with ovarian metastasis and peritoneal dissemination. Tumour Biol 2013;34:463-9. [PubMed]
- Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol 2010;17:2370-7. [PubMed]
- Hall JJ, Loggie BW, Shen P, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointest Surg 2004;8:454-63. [PubMed]
- Yonemura Y, Kawamura T, Bandou E, et al. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg 2005;92:370-5. [PubMed]
- Scaringi S, Kianmanesh R, Sabate JM, et al. Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: a single western center experience. Eur J Surg Oncol 2008;34:1246-52. [PubMed]
- Yonemura Y, Fujimura T, Nishimura G, et al. Effects of intraoperative chemohyperthermia in patients with gastric cancer with peritoneal dissemination. Surgery 1996;119:437-44. [PubMed]
- Yonemura Y, Fujimura T, Fushida S, et al. Hyperthermo-chemotherapy combined with cytoreductive surgery for the treatment of gastric cancer with peritoneal dissemination. World J Surg 1991;15:530-5; discussion 535-6. [PubMed]
- Gold JS, Jaques DP, Bentrem DJ, et al. Outcome of patients with known metastatic gastric cancer undergoing resection with therapeutic intent. Ann Surg Oncol 2007;14:365-72. [PubMed]
- Fukagawa T, Katai H, Saka M, et al. Significance of lavage cytology in advanced gastric cancer patients. World J Surg 2010;34:563-8. [PubMed]
- Mezhir JJ, Shah MA, Jacks LM, et al. Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol 2010;17:3173-80. [PubMed]
- Koga S, Hamazoe R, Maeta M, et al. Prophylactic therapy for peritoneal recurrence of gastric cancer by continuous hyperthermic peritoneal perfusion with mitomycin C. Cancer 1988;61:232-7. [PubMed]
- Hamazoe R, Maeta M, Kaibara N. Intraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer. Final results of a randomized controlled study. Cancer 1994;73:2048-52. [PubMed]
- Fujimura T, Yonemura Y, Muraoka K, et al. Continuous hyperthermic peritoneal perfusion for the prevention of peritoneal recurrence of gastric cancer: randomized controlled study. World J Surg 1994;18:150-5. [PubMed]
- Sautner T, Hofbauer F, Depisch D, et al. Adjuvant intraperitoneal cisplatin chemotherapy does not improve long-term survival after surgery for advanced gastric cancer. J Clin Oncol 1994;12:970-4. [PubMed]
- Ikeguchi M, Kondou A, Oka A, et al. Effects of continuous hyperthermic peritoneal perfusion on prognosis of gastric cancer with serosal invasion. Eur J Surg 1995;161:581-6. [PubMed]
- Rosen HR, Jatzko G, Repse S, et al. Adjuvant intraperitoneal chemotherapy with carbon-adsorbed mitomycin in patients with gastric cancer: results of a randomized multicenter trial of the Austrian Working Group for Surgical Oncology. J Clin Oncol 1998;16:2733-8. [PubMed]
- Fujimoto S, Takahashi M, Mutou T, et al. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer 1999;85:529-34. [PubMed]
- Shimoyama S, Shimizu N, Kaminishi M. Type-oriented intraoperative and adjuvant chemotherapy and survival after curative resection of advanced gastric cancer. World J Surg 1999;23:284-91; discussion 291-2. [PubMed]
- Yonemura Y, de Aretxabala X, Fujimura T, et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastroenterology 2001;48:1776-82. [PubMed]
- Miyashiro I, Furukawa H, Sasako M, et al. Randomized clinical trial of adjuvant chemotherapy with intraperitoneal and intravenous cisplatin followed by oral fluorouracil (UFT) in serosa-positive gastric cancer versus curative resection alone: final results of the Japan Clinical Oncology Group trial JCOG9206-2. Gastric Cancer 2011;14:212-8. [PubMed]
- Kuramoto M, Shimada S, Ikeshima S, et al. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg 2009;250:242-6. [PubMed]
- Blagoev KB, Wilkerson J, Stein WD, et al. Therapies with diverse mechanisms of action kill cells by a similar exponential process in advanced cancers. Cancer Res 2014;74:4653-62. [PubMed]
- Coccolini F, Cotte E, Glehen O, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol 2014;40:12-26. [PubMed]
- Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008;15:2426-32. [PubMed]
- Hagiwara A, Takahashi T, Kojima O, et al. Prophylaxis with carbon-adsorbed mitomycin against peritoneal recurrence of gastric cancer. Lancet 1992;339:629-31. [PubMed]
- Takahashi T, Hagiwara A, Shimotsuma M, et al. Prophylaxis and treatment of peritoneal carcinomatosis: intraperitoneal chemotherapy with mitomycin C bound to activated carbon particles. World J Surg 1995;19:565-9. [PubMed]
- Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575-81. [PubMed]
- Königsrainer I, Horvath P, Struller F, et al. Initial clinical experience with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in signet-ring cell gastric cancer with peritoneal metastases. J Gastric Cancer 2014;14:117-22. [PubMed]
- Smeenk RM, Verwaal VJ, Zoetmulder FA. Learning curve of combined modality treatment in peritoneal surface disease. Br J Surg 2007;94:1408-14. [PubMed]
- Franko J, Ibrahim Z, Gusani NJ, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer 2010;116:3756-62. [PubMed]
- Yan TD, Welch L, Black D, et al. A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignancy peritoneal mesothelioma. Ann Oncol 2007;18:827-34. [PubMed]
- Tewari D, Java JJ, Salani R, et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncol 2015;33:1460-6. [PubMed]
- Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet 1997;350:1647-54. [PubMed]
- Jaques DP, Coit DG, Hajdu SI, et al. Management of primary and recurrent soft-tissue sarcoma of the retroperitoneum. Ann Surg 1990;212:51-9. [PubMed]
- Lim SJ, Cormier JN, Feig BW, et al. Toxicity and outcomes associated with surgical cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC) for patients with sarcomatosis. Ann Surg Oncol 2007;14:2309-18. [PubMed]
- Baumgartner JM, Ahrendt SA, Pingpank JF, et al. Aggressive locoregional management of recurrent peritoneal sarcomatosis. J Surg Oncol 2013;107:329-34. [PubMed]
- Bryan ML, Fitzgerald NC, Levine EA, et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in sarcomatosis from gastrointestinal stromal tumor. Am Surg 2014;80:890-5. [PubMed]
- Luton JP, Cerdas S, Billaud L, et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med 1990;322:1195-201. [PubMed]
- Plager JE. Carcinoma of the adrenal cortex: clinical description, diagnosis, and treatment. Int Adv Surg Oncol 1984;7:329-53. [PubMed]
- Haq MM, Legha SS, Samaan NA, et al. Cytotoxic chemotherapy in adrenal cortical carcinoma. Cancer Treat Rep 1980;64:909-13. [PubMed]
- Schlumberger M, Ostronoff M, Bellaiche M, et al. 5-Fluorouracil, doxorubicin, and cisplatin regimen in adrenal cortical carcinoma. Cancer 1988;61:1492-4. [PubMed]
- Fassnacht M, Terzolo M, Allolio B, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med 2012;366:2189-97. [PubMed]
- Hughes M. Phase II Trial of Surgical Resection and Heated Intraperitoneal Peritoneal Chemotherapy (HIPEC) for Adrenocortical Carcinoma. ClinicalTrials.gov [Internet]. National Library of Medicine (US). 2000 - May 23, 2015. Available online: http://clinicalstudies.info.nih.gov/cgi/detail.cgi?A_2013-C-0114.html. 2015.


