Percutaneous ablation of colorectal lung metastases
Introduction
Lung metastasectomy can prolong survival in patients with metastatic colorectal carcinoma. Reported median survival ranges from 35-50 months (1,2). Reported 5-year survival rates after surgical resection range from 36-67.8% (3-5). However, many patients are considered ineligible for surgical metastasectomy due to medical co-morbidities or prior metastasectomy, rendering further resection technically challenging. Furthermore, with the possibility of additional future metastases, lung function preservation and quality of life should be a priority. In all of these clinical scenarios, thermal ablation offers a potential solution with similar reported median survival ranging from 33-46 months (6-8). It has minimal effect on pulmonary function or quality of life (9), can be repeated, and may be considered more acceptable to patients because of the associated short hospital stay and post-procedure recovery. Lastly, chemotherapy treatment does not need to be interrupted while thermal ablation is performed (Figure 1).
Heat-based ablation was first described in normal liver in 1990 using a modified Bovie knife and radiofrequency energy (10,11). Subsequent descriptions of successful thermal liver tumor ablation prompted further study of using the technique in other organs (12,13). Its successful use in lung tumors in humans was described in patients with inoperable non-small cell lung carcinoma in 2000 (14). These initial studies included a heterogeneous patient population with primary and secondary lung malignancies but provided the initial impetus to study thermal ablation as a safe method of treating inoperable lung tumors and metastases. Radiofrequency ablation (RFA) has since been joined by other heat based therapies: microwave and cryoablation.
Mechanism of action
Each ablation technique mechanism of action is described below in brief. For a more detailed description, the interested reader is directed to the following review articles on the topic (15-17).
During RFA, molecular friction is created when an electrical current is delivered to tumor cells surrounding the RFA probe tip, thus creating a rise in tissue temperature named the Joule effect (15). A temperature at the electrode tip above 60 °C will achieve cell death (18,19). Tissue surrounding the electrode is heated by electrical conduction. The adjacent tissue is then heated by thermal conduction. Thermal conduction is impeded by tissue charring which can occur if ablation temperatures are too high (e.g., above 95 °C). Tissue charring decreases ablation effectiveness (20-22). In order to avoid tissue charring, technical adaptations have been made to ablation probes including the use of multiple ablation probes (23), internally cooled probes (24) and pulsed RFA ablation systems (25).
When microwave energy is applied to human tissue, water molecules in the tissue adjacent to the probe tip continuously realign with the applied field leading to an increase in local tissue temperatures (26). Tissue destruction occurs when tissues are heated to lethal temperatures. Temperature at the probe tip can be as high as 150 °C. Microwave power penetrates tissues of low electric conductivity such as lung and charred tissue, the latter feature confers an advantage over RFA in the lungs.
Cryoablation involves rapid tumor cooling causing cell death at temperatures in the range of −50 °C. This occurs as a result of the rapid expansion of argon released from the ablation probe, named the Joule-Thompson effect, causing cooling of the adjacent tissues. Sequential warming and cooling augments the degree of cellular damage (17,27). The ice ball that forms during freezing is visible on computed tomography (CT), allowing the operator to closely monitor the ablation zone (28).
Indications for thermal ablation of colorectal lung metastases
Patients with colorectal cancer lung oligometastases can benefit from surgical metastasectomy (3-5). Similarly, these patients benefit from curative thermal ablation. Patients with tumor size smaller than 3 cm (29) and a favorable biologic profile are shown to have a lower rate of local tumor progression and cancer specific survival after thermal ablation than those with tumors larger than 3 cm and unfavorable biologic profile, including Ki67 positivity, a marker for cell proliferation (30). The 2012 Cardiovascular and Interventional Radiological Society of Europe (CIRSE) standards of practice based on expert consensus (31) state that the maximum number of lung metastases that may be ablated is not clearly defined with most centers treating patients with five or fewer metastases. Combination therapies are suggested as an option for improving chance of cure and sparing lung function including thermal ablation combined with surgery (32) or chemotherapy (33). Patients should be assessed by an interdisciplinary team and the maximum tumor diameter should not exceed 3 cm in diameter (31). A recent expert consensus document suggests that if curative ablation is intended, a tumor number cutoff of ≤3 in the case of unilateral metastases and ≤5 in the case of bilateral metastases be employed, echoing the CIRSE guidelines which suggest a cutoff of five metastases in total (31). Finally, the guideline suggests that no metastasis should be greater than 3 cm if multiple (34).
One interesting application of thermal ablation has been to render the patient free of active disease and thus permit a chemotherapy holiday while receiving rigorous imaging surveillance. Such chemotherapy breaks are reported to be possible without disease progression for durations of up to 20 months (35). An approach previously described in the case of thermal ablation of liver metastases is known as the “test of time” and may have an application in lung metastasis ablation (36). Selected resectable patients with limited disease suitable for thermal ablation undergo percutaneous ablation and imaging surveillance with the intention to undergo metastasectomy if thermal ablation does not lead to tumor control. This delayed period allows the biology of disease to declare itself. Patients completely treated by ablation, in addition to those who develop unresectable metastases under surveillance, are spared unnecessary surgery (37).
Relative contraindications for treatment include underlying interstitial disease, such as pulmonary fibrosis due to the risk of severe pulmonary failure, and coagulopathies (38-41). Lesions within 1 cm of sensitive structures such as the trachea, main bronchi, esophagus and central vessels are at higher risk of complications and may often be incompletely ablated due to heat sink effect (42).
Complications
Major complications as a result of thermal ablation are rare. Complications after lung RFA are reported to occur in 9.8% of patients; the most frequently reported complications include pleuritis, pneumonia, abscess, hemorrhage, and refractory pneumothorax requiring pleurodesis (40). Major complications are rare and include bronchopleural fistula, tumor seeding, and nerve (0.3%) (43) or diaphragmatic injury. Self-limiting rib fractures after ablation of tumor located close to the chest wall has a reported incidence of 13.5% of patients treated with RFA and MWA (44). Similar complication rates including self-limiting pneumothorax and pneumothorax requiring chest tube insertion are described after microwave and cryoablation (45). Skin burns are rare with an incidence of 0.3% (45). In terms of lung function, Lencioni and colleagues report a mild change at 3 month’s follow-up in forced vital capacity (FVC) of +13.7%, and forced expiratory volume in 1 second (FEV1) of −5.4% (9). This compares well to the surgical metastasectomy literature: one study of 27 patients after surgical resection of metastases describes a mild loss of pulmonary function including a decline in FVC of −15.2% and FEV1 of −16.3% (46).
The reported mortality rate after lung tumor RFA is 0.4% (47) and is slightly less than that reported after surgical metastasectomy (1.4-2.4%) (48). Careful patient selection can minimize the risk of complications. For example, in the largest case series reporting complications after RFA, previous systemic chemotherapy was a risk factor for pleuritis, while prior external beam radiotherapy and advanced age were risk factors for pneumonia (49). Patients with emphysema have a greater predilection for lung abscess and refractory pneumothorax. Serum platelet count (≤180,000 cells/µL) and tumor size >3 cm are risk factors for hemorrhage (49).
Reported outcomes
The literature analyzing outcomes after thermal ablation of colorectal metastases is limited by study size and patient heterogeneity. The majority of published literature describes outcomes after RFA of colorectal carcinoma lung metastases (4,6,7,29,35). Two studies report outcomes in patients after cryoablation and microwave respectively, although the latter does not report specific outcomes for patients with colorectal carcinoma lung metastases (8,50). Allowing for these limitations, the findings of 7 studies analyzing outcomes of 417 patients over a mean follow-up period range of 9-40 months after ablation of colorectal cancer lung metastases (Table 1) report 1-year survival ranging from 83.9-95%, and 3-year survival ranging from 46-59.6% (6,8,9,29,35). A 5-year survival of 34.9% is reported by one group (6). Reported median survival ranges from 33-46 months (6-8,29,35). Reported local recurrence rates range from 13-38% (6-8,29,35). These findings are comparable to reported surgical outcomes which state that median survival ranges from 35-50 months (1,2). Surgical 5-year survival rates after surgical resection range from 36-67.8% (3-5). Comparable outcomes after thermal ablation and surgical resection are described in the literature. In both patient groups, there are similarities in the number of metastases treated (on average 1-2 per patient) and tumor size (typically <3 cm). However, a like for like comparison is limited by the nuances of surgical versus thermal ablation techniques and the inherent differences in the selected patient populations (Table 2). For example, there is a broad variety of surgical approaches depending on tumor size and number ranging from wedge resection to pneumonectomy, whereas thermal ablation involves only one approach. Secondly, the surgical literature concentrates on 5-year survival data, reflecting the life expectancy of patients eligible for surgical intervention. In contrast, the ablation literature is more likely to report 1-3 year survival data, due to the multiple co-morbidities inherent to this patient population. Five-year survival after thermal ablation, when reported, is less favorable than that reported in the surgical literature.

Full table
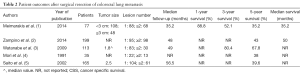
Full table
Outcome prognostic factors
In a prospective study of 293 patients with colorectal cancer lung metastases treatment with RFA, tumor size >2 cm and a number of metastases ≥3 was significantly associated with overall survival (51). Clinical factors such as the presence of treated liver metastases, and a history of prior lung surgery has no impact on survival after RFA of colorectal cancer lung metastases (7). The administration of prior or concomitant chemotherapy has a variable reported impact on outcome with one study indicating that it does not affect survival (8) and another stating that it confers a survival advantage (33). Carcinoembryonic antigen level greater than 5 ng/mL is associated with decreased local tumor progression free survival (29).
Imaging surveillance
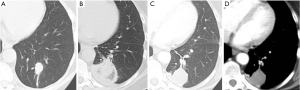
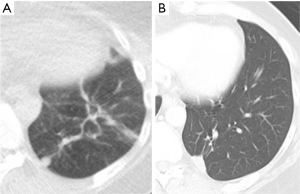
In clinical practice, PET-CT and/or CT are typically performed within two months of thermal ablation, to establish a new baseline for surveillance. In the initial imaging after ablation, the treated nodule always appears larger than the original tumor size as the ablation zone encompasses a margin around the tumor. Over time, this ablation zone should decrease in size. The patient undergoes imaging every three months for a year, and annual surveillance thereafter. An initial perilesional ground glass halo on CT during the first 2 months is likely due to inflammation which decreases over time. The expected CT appearances after 6 months include: (I) a residual nodule which is stable or decreasing in size (Figure 2); (II) an elongated linear nodule due to fibrosis; (III) atelectasis (Figure 3); or (IV) cavitation. Complete disappearance of the initial nodule is rarely observed (52).
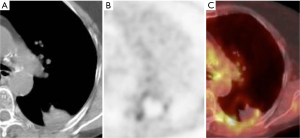
PET-CT at 24 hours and at 1 month after ablation can exhibit tracer activity at the ablation site despite adequate treatment, due to inflammation. Tracer uptake is expected to resolve by 3 months after ablation (53). The absence of tracer activity at the site of ablation on PET-CT at 6 months after ablation has been shown to correlate better with clinical outcome at 1 year than PET-CT performed within 4 days of ablation (54). The expected early PET-CT appearance is that of a relatively uniform ring of low level FDG activity (about the same as mediastinal blood pool activity) and central photopenia, a finding which may persist until 6 months and should resolve by 12 months (Figure 4) (55).
Residual disease or recurrence of disease may be present if there is contrast enhancement in the ablation zone, peripheral nodular growth, a change within the ablation zone from ground-glass to solid opacity, regional or distant lymph node enlargement, new sites of intrathoracic disease, or new extrathoracic disease; and increased metabolic activity centrally or in a nodular pattern at the ablation site on PET-CT more than 3 months after ablation (56).
Thermal ablation in combination with adjunctive therapies
Combined multimodality therapy using thermal ablation and radiation or chemotherapy may synergistically result in an improved survival compared with either modality alone. A study of 21 patients analyzing multimodality therapy reported a 3-year survival of 87.5% after multimodality therapy including RFA, radiotherapy and chemotherapy compared to 33.3% using chemotherapy alone (57).
Conclusions
Thermal ablation is an effective therapy for colorectal carcinoma lung metastases that controls disease while preserving adjacent normal lung and quality of life (9), can be repeated, and may be considered more acceptable to patients because of the associated shorter hospital stay and recovery. Patient selection is based on patient and lesion characteristics and appropriate patient selection substantially influences survival. The ideal candidate for thermal ablation has 5 metastases or fewer, with an individual lung metastasis size of <3 cm. A lesion location distant from sensitive structures and large vessels is ideal to minimize complications and avoid local recurrence, respectively. One and 3 year survival after thermal ablation of unresectable colorectal carcinoma lung metastases are good and therefore thermal ablation offers a lung-preserving therapeutic alternative to patients with medical co-morbidities.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Meimarakis G, Spelsberg F, Angele M, et al. Resection of pulmonary metastases from colon and rectal cancer: factors to predict survival differ regarding to the origin of the primary tumor. Ann Surg Oncol 2014;21:2563-72. [PubMed]
- Zampino MG, Maisonneuve P, Ravenda PS, et al. Lung metastases from colorectal cancer: analysis of prognostic factors in a single institution study. Ann Thorac Surg 2014;98:1238-45. [PubMed]
- Watanabe K, Nagai K, Kobayashi A, et al. Factors influencing survival after complete resection of pulmonary metastases from colorectal cancer. Br J Surg 2009;96:1058-65. [PubMed]
- Mori M, Tomoda H, Ishida T, et al. Surgical resection of pulmonary metastases from colorectal adenocarcinoma. Special reference to repeated pulmonary resections. Arch Surg 1991;126:1297-301, discussion 1302. [PubMed]
- Saito Y, Omiya H, Kohno K, et al. Pulmonary metastasectomy for 165 patients with colorectal carcinoma: a prognostic assessment. J Thorac Cardiovasc Surg 2002;124:1007-13. [PubMed]
- Yamakado K, Inoue Y, Takao M, et al. Long-term results of radiofrequency ablation in colorectal lung metastases: single center experience. Oncol Rep 2009;22:885-91. [PubMed]
- Gillams A, Khan Z, Osborn P, et al. Survival after radiofrequency ablation in 122 patients with inoperable colorectal lung metastases. Cardiovasc Intervent Radiol 2013;36:724-30. [PubMed]
- Yamauchi Y, Izumi Y, Hashimoto K, et al. Percutaneous cryoablation for the treatment of medically inoperable stage I non-small cell lung cancer. PLoS One 2012;7:e33223. [PubMed]
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 2008;9:621-8. [PubMed]
- McGahan JP, Browning PD, Brock JM, et al. Hepatic ablation using radiofrequency electrocautery. Invest Radiol 1990;25:267-70. [PubMed]
- Rossi S, Fornari F, Pathies C, et al. Thermal lesions induced b480 KHz localized current field in guinea pig and pig liver. Tumori 1990;76:54-7. [PubMed]
- Buscarini L, Rossi S, Fornari F, et al. Laparoscopic ablation of liver adenoma by radiofrequency electrocautery. Gastrointest Endosc 1995;41:68-70. [PubMed]
- Fraker DL. Percutaneous radiofrequency interstitial thermal ablation. Cancer J Sci Am 1995;1:30-1. [PubMed]
- Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol 2000;174:57-9. [PubMed]
- Hong K, Georgiades C. Radiofrequency ablation: mechanism of action and devices. J Vasc Interv Radiol 2010;21:S179-86. [PubMed]
- Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 2010;21:S192-203. [PubMed]
- Erinjeri JP, Clark TW. Cryoablation: mechanism of action and devices. J Vasc Interv Radiol 2010;21:S187-91. [PubMed]
- Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancies: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 2000;174:323-31. [PubMed]
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984;10:787-800. [PubMed]
- Cosman ER, Nashold BS, Ovelman-Levitt J. Theoretical aspects of radiofrequency lesions in the dorsal root entry zone. Neurosurgery 1984;15:945-50. [PubMed]
- Goldberg SN, Gazelle GS, Dawson SL, et al. Tissue ablation with radiofrequency: effect of probe size, gauge, duration, and temperature on lesion volume. Acad Radiol 1995;2:399-404. [PubMed]
- Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol 2009;38:135-43. [PubMed]
- Siperstein AE, Rogers SJ, Hansen PD, et al. Laparoscopic thermal ablation of hepatic neuroendocrine tumor metastases. Surgery 1997;122:1147-54; discussion 1154-5. [PubMed]
- Goldberg SN, Gazelle GS, Solbiati L, et al. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol 1996;3:636-44. [PubMed]
- Goldberg SN, Stein MC, Gazelle GS, et al. Percutaneous radiofrequency tissue ablation: optimization of pulsed- radiofrequency technique to increase coagulation necrosis. J Vasc Interv Radiol 1999;10:907-16. [PubMed]
- Ahmed M, Brace CL, Lee FT Jr, et al. Principles of and advances in percutaneous ablation. Radiology 2011;258:351-69. [PubMed]
- Sharma A, Moore WH, Lanuti M, et al. How I do it: radiofrequency ablation and cryoablation of lung tumors. J Thorac Imaging 2011;26:162-74. [PubMed]
- Ahrar K, Littrup PJ. Is cryotherapy the optimal technology for ablation of lung tumors? J Vasc Interv Radiol 2012;23:303-5. [PubMed]
- Yan TD, King J, Sjarif A, et al. Percutaneous radiofrequency ablation of pulmonary metastases from colorectal carcinoma: prognostic determinants for survival. Ann Surg Oncol 2006;13:1529-37. [PubMed]
- Sofocleous CT, Garg SK, Cohen P, et al. Ki 67 is an independent predictive biomarker of cancer specific and local recurrence-free survival after lung tumor ablation. Ann Surg Oncol 2013;20:S676-83. [PubMed]
- Pereira PL, Masala S. Cardiovascular and Interventional Radiological Society of Europe (CIRSE). Standards of practice: guidelines for thermal ablation of primary and secondary lung tumors. Cardiovasc Intervent Radiol 2012;35:247-54. [PubMed]
- Sano Y, Kanazawa S, Mimura H, et al. A novel strategy for treatment of metastatic pulmonary tumors: radiofrequency ablation in conjunction with surgery. J Thorac Oncol 2008;3:283-8. [PubMed]
- Chua TC, Thornbury K, Saxena A, et al. Radiofrequency ablation as an adjunct to systemic therapy for colorectal pulmonary metastases. Cancer 2010;116:2106-14. [PubMed]
- Ye X, Fan W. Minimally Invasive and Comprehensive Treatment of Lung Cancer Branch, et al. Expert consensus for thermal ablation of primary and metastatic lung tumors. Zhongguo Fei Ai Za Zhi 2014;17:294-301. [PubMed]
- Petre EN, Jia X, Thornton RH, et al. Treatment of pulmonary colorectal metastases by radiofrequency ablation. Clin Colorectal Cancer 2013;12:37-44. [PubMed]
- Livraghi T, Solbiati L, Meloni F, et al. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the “test- of-time approach”. Cancer 2003;97:3027-35. [PubMed]
- Petre EN, Sofocleous CT, Solomon SB. Ablative and Catheter-Directed Therapies for Colorectal Liver and Lung Metastases. Hematol Oncol Clin North Am 2015;29:117-33. [PubMed]
- Percutaneous radiofrequency ablation for primary or secondary lung cancers. Available online: http://www.nice.org.uk/guidance/ipg372/chapter/2-the-procedure. Accessed January 21, 2015.
- Alexander ES, Dupuy DE. Lung Cancer Ablation Technologies and Techniques. Semin Intervent Radiol 2013;30:141-50. [PubMed]
- Carrafiello G, Mangini M, Fontana F, et al. Complications of microwave and radiofrequency lung ablation: personal experience and review of the literature. Radiol Med 2012;117:201-13. [PubMed]
- Rose SC, Dupuy DE, Gervais DA, et al. Research reporting standards for percutaneous thermal ablation of lung neoplasms. J Vasc Interv Radiol 2009;20:S474-85. [PubMed]
- Bargellini I, Bozzi E, Cioni R, et al. Radiofrequency ablation of lung tumours. Insights Into Imaging 2011;2:567-76. [PubMed]
- Palussière J, Canella M, Cornelis F, et al. Retrospective review of thoracic neural damage during lung ablation - what the interventional radiologist needs to know about neural thoracic anatomy. Cardiovasc Intervent Radiol 2013;36:1602-13. [PubMed]
- Alexander ES, Hankins CA, Machan JT, et al. Rib fractures after percutaneous radiofrequency and microwave ablation of lung tumors: incidence and relevance. Radiology 2013;266:971-8. [PubMed]
- Wolf FJ, Grand DJ, Machan JT, et al. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology 2008;247:871-9. [PubMed]
- Welter S, Cheufou D, Zahin M, et al. Short- and Mid-Term Changes in Lung Function after Bilateral Pulmonary Metastasectomy. Thorac Cardiovasc Surg 2014. [Epub ahead of print]. [PubMed]
- Steinke K, Sewell PE, Dupuy D, et al. Pulmonary radiofrequency ablation—an international study survey. Anticancer Res 2004;24:339-43. [PubMed]
- Schlijper RC, Grutters JP, Houben R, et al. What to choose as radical local treatment for lung metastases from colo-rectal cancer: surgery or radiofrequency ablation? Cancer Treat Rev 2014;40:60-7. [PubMed]
- Kashima M, Yamakado K, Takaki H, et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center's experiences. AJR Am J Roentgenol 2011;197:W576-80. [PubMed]
- Vogl TJ, Naguib NN, Gruber-Rouh T, et al. Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology 2011;261:643-51. [PubMed]
- de Baère T, Aupérin A, Deschamps F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol 2015;26:987-91. [PubMed]
- Palussière J, Marcet B, Descat E, et al. Lung tumors treated with percutaneous radiofrequency ablation: computed tomography imaging follow-up. Cardiovasc Intervent Radiol 2011;34:989-97. [PubMed]
- Deandreis D, Leboulleux S, Dromain C, et al. Role of FDG PET/CT and chest CT in the follow-up of lung lesions treated with radiofrequency ablation. Radiology 2011;258:270-6. [PubMed]
- Yoo DC, Dupuy DE, Hillman SL, et al. Radiofrequency ablation of medically inoperable stage IA non-small cell lung cancer: are early post treatment PET findings predictive of treatment outcome? AJR Am J Roentgenol 2011;197:334-40. [PubMed]
- Sharma A, Lanuti M, He W, et al. Increase in fluorodeoxyglucose positron emission tomography activity following complete radiofrequency ablation of lung tumors. J Comput Assist Tomogr 2013;37:9-14. [PubMed]
- Abtin FG, Eradat J, Gutierrez AJ, et al. Radiofrequency ablation of lung tumors: imaging features of the postablation zone. Radiographics 2012;32:947-69. [PubMed]
- Inoue Y, Miki C, Hiro J, et al. Improved survival using multi-modality therapy in patients with lung metastases from colorectal cancer: a preliminary study. Oncol Rep 2005;14:1571-6. [PubMed]



