New therapeutic strategies for BRAF mutant colorectal cancers
BRAF mutation and colorectal cancers (CRCs)
BRAF, along with ARAF and CRAF, belongs to the RAF family of kinases, which are typically activated by RAS proteins and are key components of the MAPK signaling pathway (1). Oncogenic BRAF mutations occur in ~7% of human cancers, including ~10% of CRCs and ~50% of melanoma (2). Close to 90% of BRAF mutations involve a single amino acid substitution at valine 600 (V600), and nearly 90% of V600 mutations involve substitution to glutamic acid (V600E). V600 mutations lead to constitutive activation of BRAF kinase activity, leading to phosphorylation and activation of MEK kinases, MEK1 and MEK2, which in turn phosphorylate and activate ERK kinases, ERK1 and ERK2. Once activated, ERK kinases phosphorylate a number of critical cellular substrates involved in cell proliferation and survival (1,3).
In CRC, BRAF mutations are found more commonly in women, right-sided or proximal colonic tumors, and in tumors that are hypermutated or that exhibit microsatellite instability (MSI) (4-6). BRAF mutations confer poor prognosis in metastatic CRC with nearly a two-fold increase in mortality relative to patients with wild-type BRAF (7,8). BRAF V600 mutations may also predict lack of benefit from EGFR monoclonal antibodies, such as cetuximab and panitumumab. A study by Di Nicolantonio and colleagues found that in KRAS wild-type CRC patients treated with EGFR antibodies, no responses were seen in patients with BRAF V600 mutations (9). However, some larger studies have shown a potential trend toward benefit in BRAF V600 patients treated with EGFR antibody-containing regimens in the first line setting, leading some to question whether these patients might derive some benefit from these agents (10-13). Still, no study has ever shown a statistically significant survival benefit for EGFR antibodies in patients with BRAF V600 mutant CRC. Overall, due to the poor prognosis and potential resistance to standard therapies conferred by BRAF V600 mutations in CRC, new and effective therapeutic strategies are critically needed for this disease.
BRAF inhibitor insensitivity in BRAF mutant CRC
BRAF V600 mutations are also found in ~50% of melanomas. BRAF inhibitors, such as vemurafenib and dabrafenib, have produced dramatic response rates of 50-80% in BRAF mutant melanoma, revolutionizing the treatment of these cancers (14-17). Both vemurafenib and dabrafenib are FDA-approved for the treatment of BRAF mutant melanoma. However, when metastatic CRC patients harboring the same BRAF V600 mutation were treated with vemurafenib, only a 5% response rate was observed, indicating that BRAF inhibitor monotherapy is surprisingly ineffective in BRAF mutant CRC relative to BRAF mutant melanoma (18). Understanding the underpinnings of this striking disparity in sensitivity will be critical to designing effective therapies for BRAF mutant CRC.
There are several potential reasons why BRAF mutant CRCs might be less sensitive to BRAF inhibition relative to BRAF mutant melanoma. One possible explanation is that BRAF mutant CRCs may not be as dependent on MAPK signaling for proliferation and survival as BRAF mutant melanomas. This may be due to a CRC-specific lineage trait, or to the presence of alternative or parallel signaling pathways that can maintain proliferation or survival even in the absence of MAPK signaling. For example, more than 90% of CRCs harbor mutations in the Wnt/β-catenin pathway—a key regulator of cell proliferation, differentiation, and survival—most commonly through loss of the APC tumor suppressor gene (6). Interestingly, activation of the Wnt/β-catenin pathway has been implicated as a potential mechanism of resistance to BRAF inhibition in melanoma (19). It is also possible that differential activation of other key signaling pathways, such as the PI3K pathway might contribute to BRAF inhibitor resistance. Alternatively, another potential explanation is that BRAF inhibitors might not effectively suppress the MAPK signaling pathway in BRAF mutant CRC.
To understand the fundamental difference in sensitivity to BRAF inhibitors between BRAF mutant CRC and BRAF mutant melanoma, our group and others utilized BRAF mutant CRC and melanoma cell lines to model the differential effects of BRAF inhibitors (20-23). As expected, a BRAF inhibitor alone led to robust and sustained suppression of MAPK signaling in BRAF mutant melanoma cells. Surprisingly, MAPK suppression by BRAF inhibitor alone in BRAF mutant CRC cells was transient, and rapid reactivation of MAPK signaling and re-accumulation of phosphorylated ERK (P-ERK) was observed beginning roughly 6 hours after initiation of BRAF inhibitor treatment, despite continued presence of drug (21,22). Pharmacodynamic analysis of paired pre-treatment and on-treatment biopsies from BRAF mutant melanoma patients has shown that robust suppression of MAPK signaling is required for tumor response (24). Thus, incomplete MAPK pathway inhibition by BRAF inhibitors alone in BRAF mutant CRC could be a key factor, and possibly the major factor, contributing to BRAF inhibitor resistance in this disease. These findings also suggest the possibility that improved therapeutic strategies that achieve more complete inhibition of MAPK signaling in BRAF mutant CRC might be sufficient to induce meaningful clinical responses in BRAF mutant CRC patients.
A key insight into the mechanism of BRAF inhibitor resistance came from the discovery that EGFR can drive resistance and MAPK pathway feedback reactivation following BRAF inhibitor treatment in many BRAF mutant CRCs (21,23). Our group and others showed that upon BRAF inhibitor treatment, signaling from EGFR through RAS and CRAF increases, leading to re-accumulation of phosphorylated ERK (P-ERK) (Figure 1) (21,22). BRAF mutant CRCs (both in cell lines and human tumor specimens) express higher levels of total and phosphorylated EGFR (P-EGFR) than BRAF mutant melanomas, perhaps explaining why BRAF mutant CRCs are more prone to exhibit EGFR-dependent resistance and MAPK reactivation (21,23).
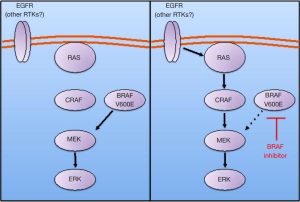
The exact mechanism by which EGFR leads to MAPK pathway reactivation is still a matter of debate. The study by Prahallad and colleagues suggested that BRAF inhibitor treatment leads to a feedback increase in the phosphorylation of EGFR (23). However, our group and others did not observe an increase in EGFR phosphorylation following BRAF inhibitor treatment (21,22). Rather, we observed an increase in the ability of EGFR to engage and activate downstream signaling effectors, such as RAS. The increase in the ability of EGFR to activate downstream signaling pathways following BRAF inhibitor treatment is hypothesized to be due to the reduction of ERK-dependent negative feedback signals, such as Sprouty proteins, that suppress the ability of receptor tyrosine kinases like EGFR to signal through the MAPK pathway (21,22). It is also possible that each of these mechanisms may predominate in different BRAF mutant CRCs, or that both mechanisms may be operant in some cancers. Regardless of the potential mechanistic differences, these studies share a consistent conclusion that EGFR is a key driver of BRAF inhibitor resistance in many BRAF mutant CRCs.
Accordingly, combinations of BRAF inhibitors and EGFR inhibitors have been evaluated in preclinical models as a potential clinical strategy to overcome EGFR driven resistance. In BRAF mutant CRC cell lines, the combination of a BRAF inhibitor and an EGFR inhibitor can lead to improved and sustained suppression of MAPK signaling and to a greater reduction in cell viability than either agent alone. Furthermore, in BRAF mutant CRC xenograft models, the combination of a BRAF and an EGFR inhibitor displayed significantly increased anti-tumor activity, in many cases leading to tumor regressions (21,23). Thus, BRAF inhibitors and EGFR inhibitors in combination represent promising components of future therapeutic strategies for this disease.
Clinical trials of BRAF inhibitor combinations for BRAF mutant CRC
Initial attempts to devise more effective clinical strategies for BRAF mutant CRC have focused on combining BRAF inhibitors with other targeted inhibitors in an effort to overcome key resistance signals. Recent clinical trials with these BRAF inhibitor combinations have produced encouraging preliminary efficacy, suggesting that this approach may represent a promising therapeutic avenue for this disease (Table 1).

Full table
BRAF + MEK inhibitor combinations
The first BRAF inhibitor combination trial for BRAF mutant CRC involved the combination of the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib (25). This trial opened in early 2011, prior to the discovery of EGFR as a major driver of resistance in BRAF mutant CRC. The rationale for this trial was based on the finding that the combination of a BRAF inhibitor and a MEK inhibitor could produce more potent and sustained suppression of MAPK signaling in BRAF mutant CRC cells, leading to increased efficacy (20). The combination of dabrafenib and trametinib has been studied extensively in BRAF mutant melanoma patients and was found to be well-tolerated and led to significantly improved response rate, progression-free survival, and overall survival relative to dabrafenib alone (31-33). In early 2014, dabrafenib and trametinib in combination received accelerated FDA approval for metastatic or unresectable BRAF mutant melanoma. Given the success of this combination in BRAF mutant melanoma, combined BRAF + MEK inhibition was hypothesized to be a promising approach to suppress multiple potential mechanisms of MAPK pathway reactivation in BRAF mutant CRC, possibly leading to improved efficacy.
Overall 43 patients with BRAF V600 mutant CRC were enrolled and received 150 mg of dabrafenib twice daily and 2 mg of trametinib daily (25). Five (12%) of patients achieved a partial response (PR) or better (confirmed and unconfirmed), including one patient who achieved a durable complete response (CR) that remains ongoing for more than 3 years. In addition, 22 (51%) of patients achieved stable disease (SD), including 11 (26%) patients who achieved a minor response. Ten (23%) patients remained on study for more than 6 months.
While the efficacy of dabrafenib and trametinib in BRAF mutant CRC was far less than that observed in BRAF mutant melanoma, this study still represented an important incremental advance in therapeutic efficacy with meaningful clinical activity observed in a subset of patients. Moreover, this study also served as a critical proof-of-concept that the MAPK pathway is a valid clinical target in BRAF mutant CRC and that effective targeting of this pathway could lead to clinical benefit in some patients.
Why is the efficacy of combined BRAF + MEK inhibition in BRAF mutant CRC still less than the efficacy of BRAF inhibitors alone in BRAF mutant melanoma? Some mechanistic clues can be derived from pharmacodynamic analysis of on-treatment tumor biopsies from patients treated with this combination. Paired pre-treatment and on-treatment biopsies taken at day 15 from nine patients from this study were evaluated. Interestingly, all patients showed a decrease in P-ERK in their on-treatment biopsies relative to matched pre-treatment biopsies. However, the mean decrease in P-ERK in BRAF mutant CRC patients treated with dabrafenib and trametinib was only 47%, compared to a mean decrease of 76% observed in BRAF mutant melanoma patients treated with dabrafenib alone. These results suggest that, even with dual MAPK pathway blockade, the degree of MAPK suppression in BRAF mutant CRC achieved by dabrafenib and trametinib may still be suboptimal. Thus, it is possible that feedback signals in BRAF mutant CRC that lead to MAPK pathway reactivation in the presence of BRAF inhibitors may be able to sustain MAPK activity despite combined BRAF and MEK inhibition. Since studies in BRAF mutant melanoma have suggested that near complete suppression of MAPK signaling is required for clinical response (24), this residual degree of MAPK pathway activity may be a major factor limiting efficacy.
BRAF + EGFR inhibitor combinations
The identification of EGFR as a critical driver of BRAF inhibitor resistance in BRAF mutant CRC has led to the development of several clinical trials evaluating combinations of BRAF and EGFR inhibitors (21,23). Most of these trials were initiated in late 2012 or early 2013, so only preliminary safety and efficacy data is available at this time. However, so far these combinations appear to be well-tolerated, and many of these approaches are showing promising initial results.
The VE-BASKET study is evaluating the combination of the BRAF inhibitor vemurafenib and the anti-EGFR monoclonal antibody cetuximab in BRAF V600 mutant CRC patients (26). As of the most recent update, 27 patients have been treated with vemurafenib and cetuximab, with 2 (7%) patients achieving a PR and 14 (52%) achieving SD. However, at the time these data were presented, most patients had only undergone one or fewer restaging assessments, so longer follow-up will be needed to assess the activity of this combination.
Results of a pilot study of vemurafenib and the anti-EGFR monoclonal antibody panitumumab in BRAF mutant CRC patients were also recently reported (27). Fifteen patients were treated, and two (13%) patients achieved an objective tumor response.
The combination of the BRAF inhibitor encorafenib (LGX818) and cetuximab is also being evaluated in BRAF mutant CRC patients (28,34). As of the most recent update, 26 patients have been treated with encorafenib and cetuximab, with a response rate of 23%. An additional 50% of patients achieved SD.
Preliminary data was also recently presented for an ongoing study of the BRAF inhibitor dabrafenib and the anti-EGFR monoclonal antibody panitumumab in BRAF V600 mutant CRC patients (29). Of the first 15 patients treated with this combination, 2 (13%) patients achieved a PR. Additionally, 11 (73%) patients achieved stable disease.
Overall, initial experience with BRAF + EGFR inhibitor combinations suggests a promising improvement in efficacy over BRAF inhibition alone, with some studies showing increased response rates and high rates of stable disease. However, a substantial percentage of patients still fail to respond to therapy, and efforts are currently underway to understand why and to develop strategies with broader efficacy.
Why do some patients fail to respond to BRAF + EGFR inhibitor combinations? Preclinical studies defining the role of EGFR in BRAF mutant CRC show a strong dependence on EGFR signaling and marked tumor regressions to combined BRAF + EGFR inhibition in some models of BRAF mutant CRC (21,23), but it is not clear whether this strong dependence on EGFR is inherent to all BRAF mutant CRCs. An initial assessment of human BRAF mutant CRCs showed that perhaps only about half of these cancers show elevated levels of P-EGFR relative to BRAF mutant melanoma (21). This finding suggests that perhaps some BRAF mutant CRCs exhibit resistance to BRAF inhibitor monotherapy through an EGFR-dependent mechanism, while others might exert resistance through EGFR-independent mechanisms. Indeed, at least one BRAF mutant CRC cell line model has been found to exhibit resistance to BRAF through signals from a different receptor tyrosine kinase (RTK), MET (35). Additionally, BRAF amplification, a known mechanism of de novo and acquired resistance to BRAF inhibitors has also been identified at baseline in some BRAF mutant CRCs (20). Thus, resistance to BRAF inhibition, and perhaps reactivation of MAPK signaling following BRAF inhibition, may be driven by EGFR-independent mechanisms in a substantial percentage of BRAF mutant CRCs. Some evidence for this possibility can be derived from pharmacodynamic assessment of paired pre-treatment and day 15 on-treatment biopsies from BRAF mutant CRC patients treated with dabrafenib and panitumumab (29). Overall, patients exhibited only a 12% mean decrease in P-ERK levels was observed. However, a closer analysis of the data reveals that a marked decrease in P-ERK levels was observed in about half of patients after initiation of therapy, whereas the other half of patients showed no decrease, or even a slight increase in P-ERK on treatment, suggesting that sustained MAPK signaling may be EGFR-dependent in some BRAF mutant CRCs, but independent of EGFR in others. Therefore, in order to develop a more effective treatment for BRAF mutant CRC, it may be necessary to target both EGFR-dependent and EGFR-independent resistance mechanisms.
Triple targeted inhibitor combinations
One approach to targeting both EGFR-dependent and EGFR-independent resistance mechanisms in BRAF mutant CRC involves combining an additional targeted inhibitor to the BRAF + EGFR inhibitor combination backbone. One such strategy builds off the initial promising efficacy observed with combined BRAF + MEK inhibition with the combination of dabrafenib and trametinib, discussed above. Since MEK inhibitors act downstream of BRAF, adding a MEK inhibitor to the combination of a BRAF and an EGFR inhibitor may allow better MAPK inhibition, expanding efficacy in cancers where EGFR is the dominant receptor reactivated with RAF inhibition and potentially in cancers with EGFR-independent resistance mechanisms. Initial results from 15 BRAF mutant CRC patients treated with the triple combination of dabrafenib, panitumumab, and trametinib showed an initial response rate of 40%, with an additional 40% of patients achieving stable disease (29). This response rate of 40% for the triple combination compares favorably to the response rates observed with each double combination—13% for dabrafenib and panitimumab, and 12% for dabrafenib and trametinib—though a head-to-head randomized comparison has not been undertaken. Still, the magnitude of the response rate difference suggests that the triple combination, which is also well-tolerated, can induce tumor responses in a larger percentage of patients than each doublet strategy alone.
Pharmacodynamic analysis of paired pre-treatment and day 15 on-treatment biopsies obtained from BRAF mutant CRC patients treated with this triple combination offers a potential mechanistic explanation for this apparent increase in efficacy. While treatment with dabrafenib + pantinumumab alone and dabrafenib + trametinib alone led to a 12% and 47% mean decrease in P-ERK respectively, the triple combination of dabrafenib + panitumumab + trametinib led to a reduction in P-ERK levels in all patients with a mean decrease of 69%, comparable to the mean 76% decrease observed in BRAF mutant melanoma patients treated with dabrafenib alone (29). Thus, more robust suppression of MAPK signaling and pathway inhibition in a large percentage of patients may account for some of the increased efficacy of the triple combination relative to each individual double combination. Overall, combined BRAF + EGFR + MEK inhibition remains a very promising approach that is undergoing continued evaluation in BRAF mutant CRC patients.
A second triple targeted inhibitor combination has also been evaluated in BRAF mutant CRC patients, involving the addition of a PI3 kinase (PI3K) alpha specific inhibitor alpelisib (BYL719) to the BRAF inhibitor encorafenib and the EGFR antibody cetuximab. The rationale for this combination is based on observations that some BRAF mutant CRCs show an upregulation of PI3K signaling following BRAF inhibitor treatment, which may or may not be mediated by EGFR (21,23). As of the most recent update, in the first 28 patients treated, this triple combination has produced a response rate of 25%, with an additional 60% of patients achieving stable disease (28,34). These numbers appear comparable to the 23% response rate seen with encorafenib and cetuximab alone (without alpelisib), though sample sizes are small. While a randomized comparison of encorafenib + cetuximab versus encorafenib + cetuximab + alpelisib is ongoing, currently there is no compelling evidence that the addition of an PI3K-alpha specific inhibitor to the combination of a BRAF and EGFR inhibitor increases efficacy in BRAF mutant CRC patients.
Combinations with cytotoxic chemotherapy
An additional strategy to increase the activity of BRAF inhibitor combinations in BRAF mutant CRC involves combinations with standard cytotoxic chemotherapy. The first such trial is evaluating the combination of vemurafenib and cetuximab in combination with irinotecan, a standard second-line chemotherapy for CRC. Initial results in nine patients show a response rate of 44% with an additional 44% of patients achieving stable disease (30). Previously, attempts to combine targeted therapies with standard cytotoxic chemotherapy has had varied success. Thus, the initial promising efficacy data seen in this study is encouraging, and suggests that other BRAF inhibitor combinations may combine effectively with chemotherapy.
Future approaches for BRAF mutant CRC
Other targeted therapy strategies
Given the promising efficacy observed with newer BRAF inhibitor combinations, additional targeted therapy combinations designed to block key resistance signals to BRAF inhibitors or that can overcome common acquired resistance mechanisms to the BRAF inhibitor combinations discussed above represent promising strategies for future development.
ERK inhibitors represent a therapeutic class of agents under current clinical development that is likely to have an important role in future targeted therapy combinations for BRAF mutant CRC. ERK inhibitors block MAPK signaling downstream of BRAF and MEK. Interestingly, preclinical studies have shown that ERK inhibitors can overcome many mechanisms that lead to reactivation of MAPK signaling and resistance to BRAF inhibitors, MEK inhibitors, or BRAF + MEK inhibitor combinations in BRAF mutant melanomas and CRCs, including RAS mutation or amplification, BRAF splice variants, BRAF amplification, or MEK1 and MEK2 mutations (36,37). Indeed, in an initial study characterizing the acquired resistance mechanisms that occur clinically in BRAF mutant CRC patients treated with BRAF inhibitor combinations (either BRAF + MEK or BRAF + EGFR inhibitor combinations), our group found that all three resistance mechanisms identified led to reactivation of MAPK signaling, but that an ERK inhibitor, either alone or in combination with a BRAF inhibitor, retained the ability to suppress MAPK signaling and could overcome resistance (38). Thus, as ERK inhibitors progress through clinical development, the evaluation of these agents either alone, or more likely in combination with BRAF and/or EGFR inhibitors may be a promising approach for BRAF mutant CRC.
Even though preclinical studies and clinical experience to date suggests that the MAPK pathway is a critical target in BRAF mutant CRC, it is also possible that other MAPK-independent pathways play an important role in BRAF mutant CRC. Therefore, the identification of alternative pathways that can be targeted together with BRAF inhibitors or BRAF inhibitor combinations (such as BRAF + EGFR inhibition) may also represent a promising avenue of exploration. For example, almost all CRCs display activation of the Wnt/β-catenin pathway (6). Thus, targeting the Wnt pathway in combination with the MAPK pathway would be a logical approach. However, in most CRCs the Wnt pathway is activated by loss of function of the APC tumor suppressor gene, leading to stabilization and accumulation of β-catenin, which drives transcription of pathway target genes. Unfortunately, the Wnt pathway has proven difficult to target with small molecules when it is activated at this downstream point, though efforts to design effective inhibitors continue (39). In some cases, though, the Wnt pathway can be activated by alterations acting upstream in the pathway at sites more amenable to pharmacologic blockade. In fact, APC mutations tend to occur less frequently in hypermutated CRCs, which is the subtype of CRC in which BRAF mutations are typically found (40). In particular, it was recently found that loss of function mutations in RNF43 occur in ~18% of CRCS and occur in a mutually exclusive fashion with APC mutations (41). These alterations lead to activation of the pathway at the level of the Wnt ligand, and thus are potentially targetable by pharmacologic inhibitors. RNF43 alterations were found to associate preferentially with tumors with microsatellite instability, a feature that is commonly associated with BRAF mutation (4,5,41). Therefore, in CRCs that harbor both BRAF V600 mutations and RNF43 alterations, co-targeting of ligand-dependent Wnt pathway activation in combination with BRAF or BRAF + EGFR inhibition represents an intriguing potential therapeutic strategy.
Immunotherapy
In the past few years, immune checkpoint inhibitors targeting the programmed death-1 (PD-1) pathway—targeting either PD-1 or PD-1 ligand 1 (PD-L1) have shown clinical promise, demonstrating the ability to induce durable regressions in melanoma, renal cell carcinoma, and non-small cell lung cancer (42-45). In contrast to the above tumor types, the efficacy of these agents so far in CRC has been limited (42-44). However, recent studies suggest that the subset of CRCs which exhibit microsatellite instability (MSI) may be especially good candidates for immune checkpoint inhibitors (46). These tumors carry a high mutational load, which creates the potential for increased burden of tumor neoantigens and show increased tumor infiltration of activated CD8-postive cytotoxic T cells and T helper type 1 (Th1) cells. Additionally, multiple immune checkpoint proteins, such as PD-1, PD-L1, and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) are found to be upregulated in MSI CRCs relative to CRCs that are microsatellite stable (MSS). Since BRAF mutations in CRC are highly correlated with MSI (4,5), there is clear rationale for the evaluation of immune checkpoint inhibitors in BRAF mutant CRC. Furthermore, studies in BRAF mutant melanoma have suggested that MAPK inhibition with BRAF inhibitors or BRAF inhibitor combinations can lead to enhanced expression of immune checkpoint proteins like PD-L1, as well as enhanced antigen expression and increased CD8-positive T lymphocyte tumor infiltration (47-49). Consistent with these findings, the combination of BRAF inhibition and PD-1 or PD-L1 inhibitors led to enhanced response and prolonged survival in a preclinical mouse model of BRAF mutant melanoma (49). These data suggest that combining an immune checkpoint inhibitor with a BRAF inhibitor combination that can promote effective MAPK pathway inhibition in BRAF mutant CRC may be a promising approach, perhaps focusing specifically on those patients whose tumors are MSI or that carry a hypermutated phenotype.
Summary
BRAF CRC represents an aggressive subtype of CRC for which there are no effective therapies. Clinical and preclinical studies have demonstrated that BRAF inhibitor monotherapy is ineffective in BRAF mutant CRC, likely due to feedback reactivation of MAPK signaling. BRAF inhibitor combinations designed to maintain MAPK pathway suppression have been the subject of recent and ongoing clinical trials, and have shown promising improvements in activity. Initial pharmacodynamic studies have suggested that improved efficacy may be related to improved suppression of MAPK pathway signaling. MAPK pathway reactivation appears to be driven by EGFR in some, but not all BRAF mutant CRCs. Thus, the best targeted therapy approaches may involve inhibitor combinations capable of concomitant blockade of EGFR-dependent and EGFR-independent mechanisms of MAPK pathway reactivation and resistance. For example, combined BRAF + EGFR + MEK inhibition has demonstrated encouraging initial efficacy in clinical trials. In future clinical trials evaluation of combined BRAF + ERK inhibition, or perhaps BRAF + EGFR + ERK inhibition may be promising strategies based on preclinical modeling of drug resistance. In addition to pursuing strategies aimed at achieving improved MAPK pathway suppression, identifying MAPK pathway-independent targets that can be co-inhibited with BRAF or BRAF inhibitor combinations may be required, as not all BRAF mutant CRCs are necessarily dependent solely on the MAPK pathway for survival. Still, our experience with BRAF mutant melanoma and preclinical and early clinical data thus far in BRAF mutant CRC suggest that effective MAPK suppression will be paramount in this disease. Ultimately, combining the most effective BRAF inhibitor combinations with cytotoxic chemotherapy or with immunotherapy agents, such as immune checkpoint inhibitors, could lead to important therapeutic synergies not achievable with targeted therapy alone. Finally, as therapeutic strategies begin to show promising clinical activity, comprehensive correlative studies to identify potential biomarkers predicting which patients will be most likely to respond to a given therapy and studies to define common clinical mechanisms of acquired resistance to therapy will be key to refining and advancing the management of patients with this lethal subtype of CRC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has received consulting fees from GSK and Genentech.
References
- Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett 2009;283:125-34. [PubMed]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54. [PubMed]
- Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 2006;24:21-44. [PubMed]
- Barault L, Veyrie N, Jooste V, et al. Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. Int J Cancer 2008;122:2255-9. [PubMed]
- Tie J, Gibbs P, Lipton L, et al. Optimizing targeted therapeutic development: analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int J Cancer 2011;128:2075-84. [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330-7. [PubMed]
- Richman SD, Seymour MT, Chambers P, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol 2009;27:5931-7. [PubMed]
- Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2103-14. [PubMed]
- Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008;26:5705-12. [PubMed]
- Bokemeyer C, Van Cutsem E, Rougier P, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer 2012;48:1466-75. [PubMed]
- Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023-34. [PubMed]
- Peeters M, Oliner KS, Parker A, et al. Massively parallel tumor multigene sequencing to evaluate response to panitumumab in a randomized phase III study of metastatic colorectal cancer. Clin Cancer Res 2013;19:1902-12. [PubMed]
- Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011-9. [PubMed]
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010;363:809-19. [PubMed]
- Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 2012;379:1893-901. [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [PubMed]
- Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012;366:707-14. [PubMed]
- Kopetz S, Desai J, Chan E, et al. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. J Clin Oncol 2010;28:abstr 3534.
- Anastas JN, Kulikauskas RM, Tamir T, et al. WNT5A enhances resistance of melanoma cells to targeted BRAF inhibitors. J Clin Invest 2014;124:2877-90. [PubMed]
- Corcoran RB, Dias-Santagata D, Bergethon K, et al. BRAF gene amplification can promote acquired resistance to MEK inhibitors in cancer cells harboring the BRAF V600E mutation. Sci Signal 2010;3:ra84. [PubMed]
- Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov 2012;2:227-35. [PubMed]
- Montero-Conde C, Ruiz-Llorente S, Dominguez JM, et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov 2013;3:520-33. [PubMed]
- Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012;483:100-3. [PubMed]
- Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 2010;467:596-9. [PubMed]
- Corcoran RB, Atreya CE, Falchook GS, et al. Phase 1-2 trial of the BRAF inhibitor dabrafenib (D) plus MEK inhibitor trametinib (T) in BRAF V600 mutant colorectal cancer (CRC): Updated efficacy and biomarker analysis. J Clin Oncol 2014;32:abstr 3517.
- Tabernero J, Chan E, Baselga J, et al. VE-BASKET, a Simon 2-stage adaptive design, phase II, histology-independent study in nonmelanoma solid tumors harboring BRAF V600 mutations (V600m): Activity of vemurafenib (VEM) with or without cetuximab (CTX) in colorectal cancer (CRC). J Clin Oncol 2014;32:abstr 3518^.
- Yaeger R, Cercek A, O'Reilly EM, et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin Cancer Res 2015;21:1313-20. [PubMed]
- Tabernero J, van Geel R, Bendell JC, et al. Phase I study of the selective BRAFV600 inhibitor encorafenib (LGX818) combined with cetuximab and with or without the α-specific PI3K inhibitor alpelisib (BYL719) in patients with advanced BRAF mutant colorectal cancer. Eur J Cancer (Oxford, England: 1990) 2014;50:199.
- Bendell JC, Atreya CE, André T, et al. Efficacy and tolerability in an open-label phase I/II study of MEK inhibitor trametinib (T), BRAF inhibitor dabrafenib (D), and anti-EGFR antibody panitumumab (P) in combination in patients (pts) with BRAF V600E mutated colorectal cancer (CRC). J Clin Oncol 2014;32:abstr 3515.
- Hong DS, Morris VK, Fu S, et al. Phase 1B study of vemurafenib in combination with irinotecan and cetuximab in patients with BRAF-mutated advanced cancers and metastatic colorectal cancer. J Clin Oncol 2014;32:abstr 3516.
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694-703. [PubMed]
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877-88. [PubMed]
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30-9. [PubMed]
- Geel RV, Elez E, Bendell JC, et al. Phase I study of the selective BRAFV600 inhibitor encorafenib (LGX818) combined with cetuximab and with or without the α-specific PI3K inhibitor BYL719 in patients with advanced BRAF-mutant colorectal cancer. J Clin Oncol 2014;32:abstr 3514.
- Straussman R, Morikawa T, Shee K, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 2012;487:500-4. [PubMed]
- Morris EJ, Jha S, Restaino CR, et al. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discov 2013;3:742-50. [PubMed]
- Hatzivassiliou G, Liu B, O'Brien C, et al. ERK inhibition overcomes acquired resistance to MEK inhibitors. Mol Cancer Ther 2012;11:1143-54. [PubMed]
- Ahronian LG, Sennott EM, Van Allen EM, et al. Clinical Acquired Resistance to RAF Inhibitor Combinations in BRAF-Mutant Colorectal Cancer through MAPK Pathway Alterations. Cancer Discov 2015;5:358-67. [PubMed]
- Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov 2014;13:513-32. [PubMed]
- Donehower LA, Creighton CJ, Schultz N, et al. MLH1-silenced and non-silenced subgroups of hypermutated colorectal carcinomas have distinct mutational landscapes. J Pathol 2013;229:99-110. [PubMed]
- Giannakis M, Hodis E, Jasmine Mu X, et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat Genet 2014;46:1264-6. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [PubMed]
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020-30. [PubMed]
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. [PubMed]
- Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 2015;5:43-51. [PubMed]
- Frederick DT, Piris A, Cogdill AP, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res 2013;19:1225-31. [PubMed]
- Liu C, Peng W, Xu C, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res 2013;19:393-403. [PubMed]
- Cooper ZA, Juneja VR, Sage PT, et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol Res 2014;2:643-54. [PubMed]


