c-MET expression in colorectal adenomas and primary carcinomas with its corresponding metastases
Introduction
Colorectal cancer (CRC) is currently the third most common cancer type worldwide and it is still the second leading cause of cancer-related deaths worldwide despite of recent advances in neo-adjuvant chemotherapeutic regimens. It is considered the most frequent gastrointestinal malignancy (1,2). In Egypt, CRC constitutes 4.2% being the 7th in men and the 4th in women (3).
Metastatic dissemination of primary colorectal tumors is directly related to patient’s survival and is considered as the most frequent reason of treatment failure. It accounts for about 90% of all CRC deaths (4). The 5-year survival of CRC patients with lymph node metastasis (LNM) is only 30% and for those with hepatic metastasis, life expectancy is severely limited (5). Liver metastasis is one of the critical prognostic factors of CRC and 15% of patients with CRC have synchronous or metachronous liver metastases (6).
Molecular targeting on oncogene is now a new therapeutic approach under intense investigation. The identification of deregulated oncogenic pathways in colonic cancer will lead to new therapeutic options. c-MET, a tyrosine kinase receptor, is overexpressed in a subset of human epithelial malignancies (7) including colorectal (8), ovarian (9), gastric (10), breast (11), endometrial (12), nasopharyngeal (13), hepatocellular (14), and non-small cell lung carcinomas (15) as well as in lymphoma (16). Such overexpression could be the result of c-MET amplification (17).
c-MET (or MET) encodes a cell surface receptor for Hepatocyte Growth Factor/Scatter Factor (HGF/SF), which is a mesenchymal cytokine with pleiotropic effects including mitogenic, monogenic and morphogenic properties (18-20). The c-MET gene has been mapped to chromosome 7 at a subtelomeric position on the q-arm (21). c-MET is expressed in a variety of normal epithelial and endothelial cells while HGF is expressed only by cells of mesenchymal origin (22).
Activation of c-MET by HGF and its signaling pathways promotes tumor cell proliferation, migration, invasion and tumor angiogenesis as well as poor prognosis (7,20,23).
c-MET amplification and overexpression were observed in colonic adenomas and primary tumors, while less expression existed in normal colonic tissues (24). In addition, the system of c-MET and its ligand HGF had been found to play a vital role in distant metastases of CRC (25,26). However, few studies to the best of our knowledge have compared.
c-MET expression in primary CRC and distant metastases, and they have yielded conflicting results (18,27,28). Therefore, it is important to investigate the concordance of results from primary tumors and distant metastases.
This study aimed to analyze the immunohistochemical expression of c-MET in colorectal adenomas and primary colorectal carcinomas including their corresponding metastases; lymph node metastases, peritoneal deposits and liver metastases. We also investigated the correlation of c-MET expression and clinicopathological association of the studied cases.
Patients and methods
Patients
We conducted a retrospective study of colorectal adenoma and carcinoma cases attended at Minia Oncology Center (Egypt), Minia University Hospital (Egypt) and Almowasat Hospital, Almadina Almonawara (Saudi Arabia) during the period between January 2011 and October 2014. Data were extracted from the pathology reports and medical records. Only cases with available adequate tumor tissue and complete clinicopathological data were considered eligible. Patients did not receive neo-adjuvant therapy. The final number available was 23 cases of colorectal adenomas and 102 cases of colorectal carcinomas, representing the study population. For comparison, 12 sections of adjacent healthy colorectal mucosa were also examined.
Diagnosis was done by colonoscopic evaluation of patients presenting with bleeding per rectum, constipation and change in the bowel habits. Multiple biopsies were taken from any suspected lesions or unhealthy mucosa for histopathology. The lesions which were confirmed histopathologically to be colorectal carcinomas were subjected to surgical resection with safety margin together with resection of its corresponding mesentery and lymph nodes according to the lesion’s site.
Concerning adenoma cases, the age and gender of patients were recorded. The histological type; tubular, villous, tubulovillous (reviewed according to WHO criteria) (29) and the histological grade of dysplasia (low and high) were obtained from histopathology data files. As regards the carcinoma cases, clinical and pathological data were retrospectively obtained from the files of the hospital medical archive. The included clinical data were histological type of the tumor, histopathological grade, tumor infiltrating lymphocytes, invasion of colonic wall, lymph node, and distant metastasis. Forty-four cases were associated with lymph node metastases, 21 cases were associated with peritoneal dissemination, and 16 cases were associated by liver metastases. Tumor type and grade were reviewed according to the WHO criteria (29). Tumor stage was estimated by the TNM staging system (30).
For the assessment of tumor infiltrating lymphocytes, the entire H&E-stained section of the tumor representing the area with the highest degree of lymphocytic infiltration was examined at low magnification to obtain an overall assessment of the extent of infiltration which was then categorized according to the density of lymphocytic infiltration as: (I) grade 0: absent; (II) grade 1: mild infiltration (scattered lymphocyte aggregates); (III) grade 2: moderate infiltration; (IV) grade 3: marked infiltration (31,32).
Immunohistochemistry
Immunohistochemistry was performed on 10% buffered formalin fixed, paraffin embedded tissue blocks. On 4-µm tissue sections, pre-diluted rabbit anti-human c-MET monoclonal antibody (clone SP44, spring bioscience, CA, USA) was used as the primary antibody. Briefly, after deparaffinization in xylene and hydration in descending grades of alcohol till distilled water, endogenous peroxidase activity was inhibited by hydrogen peroxide. Antigens were retrieved by using 0.01 M sodium citrate and heated in a microwave oven for 20 min and then incubated with primary antibody for 30 min at room temperature. For the secondary developing reagents, a labeled streptavidin-biotin kit (Novocastra, Germany) was used. Antigens were visualized using Envision system (Novocastra, Germany) and diaminobenzidine (DAB) (Novocastra, Germany). Finally sections were counterstained with hematoxylin (Novocastra, Germany) then dehydrated and mounted. In negative control slides, primary antibody was not included. Human breast carcinoma was used as the positive control.
Evaluation of immunohistochemical staining
The specimens were evaluated independently by two of the authors (N.R. & M.F.) in a blind fashion to the clinic-pathological data. The cytoplasmic/membranous c-MET immunoreactivity in cells was evaluated by considering the intensity of staining as follow: (I) 0, negative immunostaining; (II) 1, weak immunostaining; (III) 2, moderately positive immunostaining; and (IV) 3, strongly positive immunostaining. We defined scores 0 and 1 as c-MET-low expression, and scores 2 and 3 as c-MET-high expression (19,33).
Statistical analysis
All statistical analyses were performed using SPSS 16.0 computer software. To test associations between categorical variables, Chi-square and Fisher exact tests were conducted. Kappa was used a measure of agreement between primary cancer and different metastatic sites. P of 0.05 was used as a significance criterion.
Results
Clinicopathological data of patients
Adenomas
The examined 23 adenoma cases were 15 males (65.2%) and 8 females (34.8%). The M:F ratio was 1.87:1. The mean age was (53.87±7.65) years and a median of 53 years (range, 43-69 years). Adenoma cases were of the following histological types: 15 (65.3%) tubular adenomas, 3 (13%) villous adenomas and 5 (21.7%) tubulovillous adenomas. Eleven adenomas (47.8%) showed low dysplasia, 12 adenomas (52.2%) showed high dysplasia.
Carcinomas
The mean age of the studied colorectal carcinoma cases was (51.49±13.87) years and a median of 51 years (range, 22-79 years). This study included 56 (54.9%) males and 46 (54.9%) females. The M:F ratio was 1.2:1. There were 3 (2.9%), 36 (35.3%), 8 (7.8%), 17 (16.7%), 18 (17.6%) and 20 (19.6%) cases of carcinomas located in the caecum, ascending colon, transverse colon, descending colon, sigmoid colon and rectum, respectively. According to tumor type, 76 cases (74.55%) were adenocarcinomas, 17 cases (16.7%) were mucoid carcinomas and 9 cases (8.8%) were signet ring carcinomas. Among the 76 adenocarcinoma cases, 4 cases (3.9%) were well-differentiated adenocarcinoma, 42 cases (41.2%) were moderately differentiated adenocarcinoma and 30 cases (29.4%) were poorly differentiated adenocarcinoma. Lymphocytic infiltrate was found in 58 (56.9%) of the cancer cases in which, 23 tumors (22.5%) showed mild lymphocytic infiltrate, 15 (14.7%) tumors displayed moderate lymphocytic infiltrate and 20 tumors (19.6%) had marked lymphocytic infiltrate. Regarding the stage of cases, 55 cases (53.9%) were stages I, II and 47 (36.3%) cases were stages III, IV.
c-MET expression in the study groups
Normal tissues showed either negative or weak reaction in 66.67% and 33.33% of cases respectively (Figure 1A). Adenomas had a positive rate of 47.8%, while the positive rate in cancer reached 66.7%. Statistically significant differences were present among the three groups (P=0.011; P=0.594 in normal mucosa vs. adenoma, P=0.035 in normal mucosa vs. carcinoma and P=0.030 in adenoma vs. carcinoma).
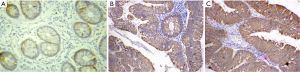
c-MET expression in adenoma
c-MET immunoreaction was confined to cytoplasm/membranous staining as shown in (Figure 1B,C). Overall, 12/23 (52.2%) of adenomas were c-MET low expression and c-MET high expression was found in 11/23 (47.8%). A significant positive association was identified between c-MET high expression and degree of dysplasia (P=0.009). The higher the degree of dysplasia, the higher the expression level of c-MET. There was no significant relation between c-MET expression and the histological type (P=0.579). Table 1 shows the association between c-MET expression and different clinicopathological parameters.
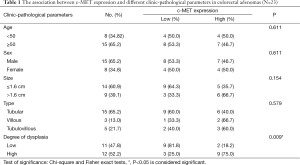
Full table
c-MET expression in primary colorectal carcinoma
c-MET immunostaining was located in the cytoplasm/membrane of tumor cells (Figure 2A-G). The c-MET high expression was found in 68 cases (66.7%) of primary CRC tumors and low expression in 34 cases (33.3%). Significant positive correlations were detected between c-MET expression and TNM stage (P=0.014), and matched lymph node and liver metastasis (P=0.038, P=0.045 respectively). There was no significant correlation between c-MET expressions with any of other clinicopathological parameters. Table 2 shows the association between c-MET expression and different clinicopathologic parameters.
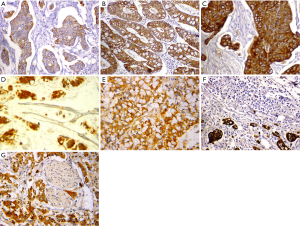
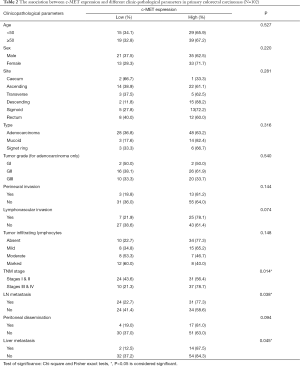
Full table
c-MET expression in matched metastasis and peritoneal dissemination
In 44 pairs of primary tumors and matched lymph node metastases, c-MET positive expression was found in 35(79.5%) and 38(86.4%) cases of primary tumors and matched lymph node metastases respectively. Only 39 cases (88.6%) positive for c-MET showed concordance result (P<0.001), Kappa=0.601 (Figure 3A,B).
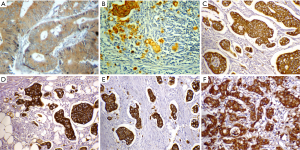
In 21 pairs of primary tumors and peritoneal dissemination, c-MET positive expression was found in 14 (66.7%) cases of primary tumors and 15 (71.4%) cases of matched peritoneal dissemination. The concordance result was found in 18 cases (85.7%) positive for c-MET (P=0.002), Kappa=0.667 (Figure 3C,D).
In 16 pairs of primary tumors and liver metastases, 13 (81.2%) cases of primary tumors and 15 (93.8%) cases of matched liver metastases showed c-MET positive expression. Fourteen cases (87.5%) positive for c-MET showed concordance result (P=0.032), Kappa=0.448 (Figure 3E,F).
Discussion
c-MET is a key driver of oncogenic transformation and is associated with poor clinical outcome in a defined subset of cancers. The poor prognosis of metastatic CRC patients underscores the importance of defining molecular factors responsible for cancer metastasis. Therefore, identification of prognostic factors allows the definition of high risk groups of patients for whom optimal therapy might be necessary (34).
In this study we examine the degree of c-MET expression in adenoma and primary colorectal carcinomas as well as its correlation with clinicopathological parameters. We also analyzed the c-MET expression in primary tumors in relation to c-MET expression in matched metastases, with special focus on regional lymph nodes, peritoneal and liver metastases.
We found that adenomas had a c-MET high expression in 47.8%, while the high expression in carcinomas reached 66.7% with statistically significant differences among the three groups; normal versus adenoma groups, normal versus carcinoma groups and in adenoma versus carcinoma groups. In this study, c-MET expression in matched metastasis and peritoneal dissemination was statistically significant. We found that c-MET overexpression in the corresponding metastatic sites including LNM, peritoneal dissemination and liver metastasis were 86.4%, 71.4%, and 93.8%, respectively. Previous studies were reported that c-MET was overexpressed in the cancer tissue when compared with its expression in corresponding normal tissue (28,35-37). Boon et al. (38) stated that along the colon adenoma-carcinoma sequence, c-MET was overexpressed in both lesions without any apparent correlation. Beside that previous studies observed a change in the expression of c-MET from primary tumors to metastases (8,33,39).
Our results has shown that the rate of c-MET expression was significantly increased with the course of cancer development as c-MET is expressed more strongly in adenoma and primary CRC than in normal mucosa and that c-MET expression in metastases is higher than that in the corresponding primary cancer. This progressive increase highlights the oncogenic role of c-MET in colorectal carcinogenesis.
In adenoma cases, our results demonstrated that c-MET overexpression was significantly related to the grade of dysplasia where c-MET overexpression was significantly higher with advanced dysplastic changes. There was no significant relation between c-MET expression and histological type. Previous studies, mentioned that, there was no significant relation between c-MET expression with histological type or grade of dysplasia of adenomas (35,40).
TNM classification is still believed to be a better option in evaluating prognosis. We found that c-MET correlated with TNM stages with a perceptible and progressive significant increase of c-MET expression from stage I to stage IV. Our data are in part corroborated by some data already published where c-MET was indeed demonstrated to be a good marker for predicting the metastatic potential of colorectal tumors (25,36,37,41). Our results coincided with the results of (37) which found that the patients with low c-MET expression had fewer nodal and distant metastases.
According to our results and previous study by Abou-Bakr and Elbasmi (37), c-MET expression can be used in a preoperative staging scheme. There are many potential benefits to include molecular markers in staging of CRC and select the group of patients with worse prognosis and who are at high risk of relapse.
There was no significant correlation between c-MET expression and clinic-pathological variables. This is in accordance with previous studies (25,28,37,42,43). However, Tabuchi et al. (42) reported that a significant difference was found between the regional LNM, venous invasion and lymphatic invasion. A trend for low c-MET expression in well differentiated cancers compared to the moderately or poorly differentiated ones was previously observed (35).
The regional lymph node and liver are common sites of metastasis in patients with CRC (44). We found that c-MET expression in metastatic tissues was also higher than that of the primary tumor. However, the difference was not significant. Concordance rate between primary tumors and lymph node metastases was 88.6%, between primary tumors and peritoneal dissemination was 85.7% and between primary tumors and liver metastasis was 87.5%. We conclude that, high concordance rates was found between primary tumors and matched metastases. Concordance rate suggests that primary tumors and their corresponding metastases had the same clone (44). Thus, metastatic cells can express most of the genes existing in their progenitors including c-MET. The difference may be due to their expression being influenced by local microenvironments of liver and lymph node.
Some previous studies have analyzed c-MET protein expression in primary CRC and metastases. Few studies showed that c-MET protein expression tended to be decreased in distant metastases compared to their corresponding primary tumors (28,45). Other studies including this one observed that c-MET expression tended to be increased in distant metastases compared to their corresponding primary tumors (8,25,33,39). Further investigation of c-MET activation in primary tumors and their corresponding metastases is needed to determine the importance of c-MET in the metastasis of CRC.
Our study is being one of the first studies to examine c-MET expression in the peritoneal deposits with correlation with their primary CRC. Our results showed that positive protein expression of c-MET in peritoneal deposits was statistically higher than corresponding primary colon. Previous studies have found that local peritoneal involvement is a strong predictor of adverse outcome in stage II and stage III disease and tumor perforation into the peritoneal cavity is a well-established adverse prognostic factor in CRC (46-48).
c-MET may serve as a biomarker for targeted therapy. Several molecules targeting c-MET have been tested in early phase clinical trials (49,50). Most of them are small kinase inhibitors, while others are biological antagonists and monoclonal antibodies targeting either the ligand or the receptor. Combination therapy with MET tyrosine kinase inhibitors and standard chemotherapeutic agents is one treatment modality that targets the HGF/MET pathway (51,52).
In conclusion, our findings suggest that c-MET appears to be an important prognostic factor for patients with CRC. Additional studies of c-MET activation and signal transduction will increase our knowledge of the role of c-MET in CRC metastasis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Parkin DM, Ferlay J, Curado MP, et al. Fifty years of cancer incidence: CI5 I-IX. Int J Cancer 2010;127:2918-27. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- El-Bolkainy MN, Nouh MA, Farahat IG, et al, editors. Pathology of Cancer. Fourth edition. Cairo: Cairo Press, 2013:205.
- Stein U, Schlag PM. Clinical, biological, and molecular aspects of metastasis in colorectal cancer. Recent Results Cancer Res 2007;176:61-80. [PubMed]
- Frati L, Codacci-Pisanelli G. Chemotherapy and immunotherapy in metastatic colorectal cancer. N Engl J Med 2009;360:2134-5. [PubMed]
- Manfredi S, Lepage C, Hatem C, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254-9. [PubMed]
- Liu X, Newton RC, Scherle PA. Developing c-MET pathway inhibitors for cancer therapy: progress and challenges. Trends Mol Med 2010;16:37-45. [PubMed]
- Sun YL, Liu WD, Ma GY, et al. Expression of HGF and Met in human tissues of colorectal cancers: biological and clinical implications for synchronous liver metastasis. Int J Med Sci 2013;10:548-59. [PubMed]
- Mhawech-Fauceglia P, Afkhami M, Pejovic T. MET/HGF signaling pathway in ovarian carcinoma: Clinical implications and future direction. Patholog Res Int 2012;2012:960327.
- Lee HE, Kim MA, Lee HS, et al. MET in gastric carcinomas: comparison between protein expression and gene copy number and impact on clinical outcome. Br J Cancer 2012;107:325-33. [PubMed]
- Raghav KP, Wang W, Liu S, et al. cMET and phospho-cMET protein levels in breast cancers and survival outcomes. Clin Cancer Res 2012;18:2269-77. [PubMed]
- Felix AS, Edwards RP, Stone RA, et al. Associations between hepatocyte growth factor, c-Met, and basic fibroblast growth factor and survival in endometrial cancer patients. Br J Cancer 2012;106:2004-9. [PubMed]
- Sun R, Zhang Q, Guo L, et al. HGF stimulates proliferation through the HGF/c-Met pathway in nasopharyngeal carcinoma cells. Oncol Lett 2012;3:1124-8. [PubMed]
- Chu JS, Ge FJ, Zhang B, et al. Expression and prognostic value of VEGFR-2, PDGFR-β, and c-Met in advanced hepatocellular carcinoma. J Exp Clin Cancer Res 2013;32:16. [PubMed]
- Landi L, Minuti G, D’Incecco A, et al. Targeting c-MET in the battle against advanced nonsmall-cell lung cancer. Curr Opin Oncol 2013;25:130-6. [PubMed]
- Xu C, Plattel W, van den Berg A, et al. Expression of the c-Met oncogene by tumor cells predicts a favorable outcome in classical Hodgkin’s lymphoma. Haematologica 2012;97:572-8. [PubMed]
- Cappuzzo F, Varella-Garcia M, Finocchiaro G, et al. Primary resistance to cetuximab therapy in EGFR FISH-positive colorectal cancer patients. Br J Cancer 2008;99:83-9. [PubMed]
- Otte JM, Schmitz F, Kiehne K, et al. Functional expression of HGF and its receptor in human colorectal cancer. Digestion 2000;61:237-46. [PubMed]
- Takeuchi H, Bilchik A, Saha S, et al. c-MET expression level in primary colon cancer: a predictor of tumor invasion and lymph node metastases. Clin Cancer Res 2003;9:1480-8. [PubMed]
- Matsumoto K, Nakamura T. Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions. Int J Cancer 2006;119:477-83. [PubMed]
- Galimi F, Torti D, Sassi F, et al. Genetic and expression analysis of MET, MACC1, and HGF in metastatic colorectal cancer: response to MET inhibition in patient xenografts and pathologic correlations. Clin Cancer Res 2011;17:3146-56. [PubMed]
- Sotoudeh K, Hashemi F, Madjd Z, et al. The clinicopathologic association of c-MET overexpression in Iranian gastric carcinomas; an immunohistochemical study of tissue microarrays. Diagn Pathol 2012;7.-57 [PubMed]
- You WK, McDonald DM. The hepatocyte growth factor/c-Met signaling pathway as a therapeutic target to inhibit angiogenesis. BMB Rep 2008;41:833-9. [PubMed]
- Dienstmann R, Serpico D, Rodon J, et al. Molecular profiling of patients with colorectal cancer and matched targeted therapy in phase I clinical trials. Mol Cancer Ther 2012;11:2062-71. [PubMed]
- Zeng ZS, Weiser MR, Kuntz E, et al. c-Met gene amplification is associated with advanced stage colorectal cancer and liver metastases. Cancer Lett 2008;265:258-69. [PubMed]
- Osada S, Matsui S, Komori S, et al. Effect of hepatocyte growth factor on progression of liver metastasis in colorectal cancer. Hepatogastroenterology 2010;57:76-80. [PubMed]
- Fujita S, Sugano K. Expression of c-met proto-oncogene in primary colorectal cancer and liver metastases. Jpn J Clin Oncol 1997;27:378-83. [PubMed]
- Isaksson-Mettävainio M, Van Guelpen B, Oberg A, et al. c-Met expression in primary tumors and their corresponding distant metastases. Mol Med Rep 2008;1:787-90. [PubMed]
- Hamilton SR, Vogelstein B, Kudo S. World Health Organization Classification of Tumors. Pathology and Genetics of Tumours of the Digestive System. Carcinoma of the colon and rectum. Lyon: IARC Press, 2000:103-43.
- Treanor D, Quirke P. Pathology of colorectal cancer. Clin Oncol (R Coll Radiol) 2007;19:769-76. [PubMed]
- Roxburgh CS, McMillan DC. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat Rev 2012;38:451-66. [PubMed]
- Richards CH, Roxburgh CS, Powell AG, et al. The clinical utility of the local inflammatory response in colorectal cancer. Eur J Cancer 2014;50:309-19. [PubMed]
- Shoji H, Yamada Y, Taniguchi H, et al. Clinical impact of c-MET expression and genetic mutational status in colorectal cancer patients after liver resection. Cancer Sci 2014;105:1002-7. [PubMed]
- Sierra JR, Tsao MS. c-MET as a potential therapeutic target and biomarker in cancer. Ther Adv Med Oncol 2011;3:S21-35. [PubMed]
- Baldus SE, Kort EJ, Schirmacher P, et al. Quantification of MET and hepatocyte growth factor/scatter factor expression in colorectal adenomas, carcinomas and non-neoplastic epithelia by quantitative laser scanning microscopy. Int J Oncol 2007;31:199-204. [PubMed]
- Kammula US, Kuntz EJ, Francone TD, et al. Molecular co-expression of the c-Met oncogene and hepatocyte growth factor in primary colon cancer predicts tumor stage and clinical outcome. Cancer Lett 2007;248:219-28. [PubMed]
- Abou-Bakr AA, Elbasmi A. c-MET overexpression as a prognostic biomarker in colorectal adenocarcinoma. Gulf J Oncolog 2013;1:28-34. [PubMed]
- Boon EM, van der Neut R, van de Wetering M, et al. Wnt signaling regulates expression of the receptor tyrosine kinase met in colorectal cancer. Cancer Res 2002;62:5126-8. [PubMed]
- Voutsina A, Tzardi M, Kalikaki A, et al. Combined analysis of KRAS and PIK3CA mutations, MET and PTEN expression in primary tumors and corresponding metastases in colorectal cancer. Mod Pathol 2013;26:302-13. [PubMed]
- Trovato M, Vitarelli E, Grosso M, et al. Immunohistochemical expression of HGF, c-MET and transcription factor STAT3 in colorectal tumors. Eur J Histochem 2004;48:291-7. [PubMed]
- De Oliveira AT, Matos D, Logullo AF, et al. MET is highly expressed in advanced stages of colorectal cancer and indicates worse prognosis and mortality. Anticancer Res 2009;29:4807-11. [PubMed]
- Tabuchi Y, Nakamura T, Ohno M, et al. Immunohistochemical Expression of c-Met (HGR Receptor) in Colorectal Cancer Lesions. Bulletin of Health Science Kobe 1998;14:43-48.
- Saigusa S, Toiyama Y, Tanaka K, et al. Inhibition of HGF/cMET expression prevents distant recurrence of rectal cancer after preoperative chemoradiotherapy. Int J Oncol 2012;40:583-91. [PubMed]
- Knösel T, Schlüns K, Dietel M, et al. Chromosomal alterations in lung metastases of colorectal carcinomas: associations with tissue specific tumor dissemination. Clin Exp Metastasis 2005;22:533-8. [PubMed]
- Matsui S, Osada S, Tomita H, et al. Clinical significance of aggressive hepatectomy for colorectal liver metastasis, evaluated from the HGF/c-Met pathway. Int J Oncol 2010;37:289-97. [PubMed]
- Kojima M, Nakajima K, Ishii G, et al. Peritoneal elastic laminal invasion of colorectal cancer: the diagnostic utility and clinicopathologic relationship. Am J Surg Pathol 2010;34:1351-60. [PubMed]
- Puppa G, Shepherd NA, Sheahan K, et al. Peritoneal elastic lamina invasion in colorectal cancer: the answer to a controversial area of pathology. Am J Surg Pathol 2011;35:465-8. [PubMed]
- Toiyama Y, Yasuda H, Saigusa S, et al. Co-expression of hepatocyte growth factor and c-Met predicts peritoneal dissemination established by autocrine hepatocyte growth factor/c-Met signaling in gastric cancer. Int J Cancer 2012;130:2912-21. [PubMed]
- Migliore C, Giordano S. Molecular cancer therapy: can our expectation be MET? Eur J Cancer 2008;44:641-51. [PubMed]
- Trusolino L, Bertotti A, Comoglio PM. MET signaling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol 2010;11:834-48. [PubMed]
- Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 2008;7:504-16. [PubMed]
- Liska D, Chen CT, Bachleitner-Hofmann T, et al. HGF rescues colorectal cancer cells from EGFR inhibition via MET activation. Clin Cancer Res 2011;17:472-82. [PubMed]


