Pain management of pancreatic head adenocarcinomas that are unresectable: celiac plexus neurolysis and splanchnicectomy
Introduction
Adenocarcinoma of the pancreas is the fifth leading cause of cancer death in the world (1). Because patients often present with locally advanced or metastatic disease, curative resection is rarely an option. As a result, intervention for these unfortunate patients is often limited to palliation. The primary goal of palliation is ensuring that patients do not suffer painful effects of cancer progression, like obstruction of the common bile duct and/or duodenum and abdominal pain from malignant infiltration into the celiac plexus.
Up to 90% of patients with pancreatic cancer experience pain (1). Narcotics may be given initially, but significant side effects, such as a reduction in quality of life, have been reported. Because of this, attention has been given to two palliative interventions: celiac neurolysis and splanchnic neurectomy. Both celiac plexus neurolysis (CPN) and splanchnicectomy have been examined and described in the literature for a number of years. The purpose of this paper is to outline pertinent anatomy, techniques, side effects, complications, and the efficacy of CPN and splanchnicectomy for palliation of pain from pancreatic cancer.
Anatomy
Pain from pancreatic cancer is believed to stem from malignant neural invasion and the stimulation of visceral afferent neural fibers which travel from the celiac plexus through the splanchnics (2). A majority of patients report pain in the epigastrium and over half of these same patients complain of associated back pain. Only a minority of patients, however, report back pain without epigastric discomfort (3).
Neurolytic treatment is directed at the celiac plexus, while a neurectomy is performed on the splanchnic nerves, either unilaterally or bilaterally. The celiac plexus is made up of the right and left ganglia, surrounding the aorta at the level of the celiac artery. It consists of visceral afferent, as well as sympathetic, and parasympathetic efferent fibers (4), and is located in the peri-aortic fat pads at the level of the diaphragmatic hiatus and celiac artery. There are commonly two to five celiac ganglia lying between T12 and L2 (5). Sympathetic nerve fibers run from the spinal cord to the sympathetic chain and then synapse in the celiac ganglia. In turn, pain from the foregut and midgut travels retrograde via parasympathetic visceral afferent nerve impulses from the celiac plexus through the splanchnic nerves to the central nervous system.
The splanchnic nerves are easily recognized as neural branches from the sympathetic trunk running anterior and inferiorly toward the diaphragmatic hiatus overlying the thoracic vertebral column. There are three classically described splanchnic nerves: the Greater, Lesser, and Least. Branches at levels T5-T9 most commonly form the greater splanchnic nerves, while the lesser splanchnics are formed from ganglia associated with T8-T12 and the least splanchnic nerves are formed by T10-L1. After being relayed from the splanchnics, stimuli reach the thalamus and cortex of the brain; this information is perceived as pain (6).
Celiac plexus neurolysis (CPN)
Originally described in 1914, modern CPN may be performed percutaneously, at the time of laparotomy, or under the direction of endoscopic ultrasound (EUS) (7). Alcohol is typically injected into the plexus but it may also be injected into the ganglia proper. Although steroid injections have been described for CPN, they are more commonly used for pain associated with chronic pancreatitis than for pain with pancreatic cancer.
Technique
Historically, percutaneous and surgical neurolysis was considered the mainstay treatment. Percutaneous CPN is generally approached posteriorly with imaging guidance, while surgical neurolysis, which was originally performed during staging laparotomy, has been replaced by laparoscopy (4,8). Over time, however, both treatments seem to have yielded to the EUS approach. EUS CPN offers several advantages over radiologic and surgical techniques, including enhanced needle precision, the ability to inject the neurolytic agent into a larger area, and the ability to perform CPN at the time of tumor biopsy and staging (9). Regardless of which technique is chosen, alcohol is injected bilaterally into the peri-aortic fat pad at the level of celiac artery and diaphragmatic hiatus.
EUS-guided CPN is currently the most common technique used today. Consistent with other endoscopic procedures, traditional preoperative questioning and positioning is performed. Next, adequate hydration is ensured and anticoagulants are held as indicated. Pulse oximetry and non-invasive blood pressure monitoring are obtained while the patient is sedated and recovering. Antibiotics are administered for those on proton pump inhibitors due to the risk of post-operative abscess from bacterial overgrowth of the upper GI tract. EUS may be performed using linear-array endosonographic imaging by way of a GF-UC30P (Olympus Corporation, Center Valley, PA, USA), GF UC140P-AL5, or GF UC 160 PAT8 (Pentax Precision Instruments, Orangeburg, NY, USA).
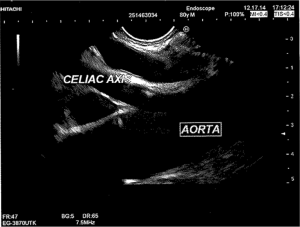
Visualization of the celiac plexus is best seen from the posterior lesser curve of the stomach. The aorta is seen longitudinally, and the first arterial branch below the diaphragm is identified (Figure 1). With experience, the celiac plexus and ganglia can be readily identified. Traditionally, a 22-guage needle is advanced through the scope after being purged of air in anticipation of injection. There are larger specialty needles for CPN, including needles with multiple side-holes, to allow for a larger injection field (EUSN-20-CPN: Cook Endoscopy, Winston-Salem, NC, USA). The needle is advanced near the lateral anterior aorta, flushed, and aspirated. For CPN in pancreatic cancer patients, 10 mL (0.25%) of bupivacaine is injected, followed by 10 mL of dehydrated (98%) alcohol. The needle is then flushed and directed to the contralateral side of the aorta where the injection sequence is repeated. Impediments to visualization include lymphadenopathy or direct tumor encasement of the plexus and/or ganglia. In these cases, unilateral injection may be the only possibility, which could result in an associated decrease in efficacy (10). This procedure typically takes well under an hour. Afterwards, the patient is monitored and then discharged home in the absence of unstable vital signs as appropriate.
Literature
Multiple studies have compared CPN to medical pain management. In 1995, Eisenberg et al. reported pain relief in 90% of their patients at 3 months from CPN, with a majority of those having significant relief until death (11). Lillemoe et al. and Wong et al. both reported pain control beyond 6 months to be common (8,12). In 2004, JAMA published a randomized control trial (RCT) that compared patients who underwent percutaneous CPN using a posterior approach with patients given systemic analgesic medications (12). Their results showed a significant difference in pain scores between the two groups, with the CPN patients reporting less severe pain (14% vs. 40%; P=0.005). This same study, however, did not show CPN to improve patient quality of life or survival. In 2007, Yan et al. performed a meta-analysis of five randomized trials comparing CPN to medical management (13). A significant difference was found between groups in visual analog scores and opioid usage, the results favored CPN. A second meta-analysis of nine RCT’s performed by Puli et al. in 2009, showed an 80% decrease in pain with CPN compared to non-interventional management (14). In a RCT by Wyse et al. [2011], patients were randomized to CPN had significantly less pain than those who did not have intra-operative neurolysis (15).
Predictive factors for failure of CPN include direct tumor invasion of the plexus and unilateral injection (10). To date, there have been no head-to-head comparisons between CPN techniques. As a result, endoscopic, percutaneous, and surgical approaches to CPN are considered equally effective.
Complications
Complications of CPN are rare, occurring in approximately 1.5-2% of patients. Possible complications, however, do include transient, usually asymptomatic hypotension, retroperitoneal abscess, and severe self-limited post-procedural pain. Transient complications include post-procedural diarrhea and hypotension due to sympathetic blockade. Permanent, unremitting diarrhea has been reported in very rare cases (16). There is also a risk of cephalic spread of the neurolytic agent, which may result in involvement of the cardiac nerves and plexus (17). Spinal complications have also been reported, particularly with posterior approaches; fortunately, these are rare, occurring in less than 1% of patients. Lower extremity weakness, paresthesias, paraplegia have all been reported. This is likely due to the alcohol injection causing spasm or thrombosis of the Artery of Adamkiewicz, which supplies the inferior spinal cord (18,19). At least one fatality has been reported from associated complications (20).
Thoracoscopic splanchnicectomy
The first description of palliative chemical splanchnicectomy dates back to 1969. The first description of bilateral splanchnicectomy for pain secondary to pancreatic cancer was described by Sadar et al. in 1974 (21,22). Splanchnicectomy was initially performed under direct vision at the time of thoracotomy and combined with sympathectomy (22). The use of the thoracoscope to aid in the performance of splanchnicectomy for palliation of pain associated with pancreatic cancer was later described in 1993 in the British Journal of Surgery (23). Since then, several short case series have been published as techniques continue to be refined. Today, thoracoscopic splanchnicectomy may be performed either unilaterally or bilaterally. Prior to consideration for splanchnicectomy, we ensure patients have failed medical management. Failure of medical managements is a subjective opinion, but if a patient’s pain is able to be controlled by fewer than three daily doses of moderate strength narcotics, and they are able to maintain a productive life, surgical management may be avoided or at least delayed. We define pain control as a patient rating his or her pain as ≤3/10 on a visual analog score, and a productive life as being able to leave one’s home and/or accomplish activities of daily living in line with the expectations of the patient. If these criteria are not met, consideration for bilateral thoracoscopic splanchnicectomy (BTS) is given.
When interviewing the patient, special attention should be given to his or her pulmonary reserve as well as to previous thoracic disease and/or interventions. Clues to possible thoracic adhesions should be explored. These include previous severe pulmonary infections with associated empyema or parapneumonic effusions, the need for previous thoracostomy drainage or thoracentesis, thoracic trauma with associated hemothorax, previous pneumothorax, and previous thoracoscopy or thoracotomy. If any of these situations apply, the patient should be informed that it may be challenging to visualize the splanchnic nerves on the affected side without extensive dissection and/or thoracotomy. In a palliative operation, these patients should be largely avoided because of increased morbidity. Counseling should also be provided on the limited but distinct possibility of continued pain despite a technically successful operation. Following appropriate preoperative discussions, the patient is consented for the procedure.
Technique
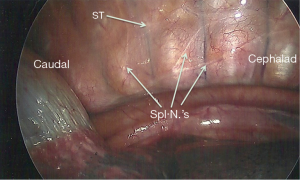
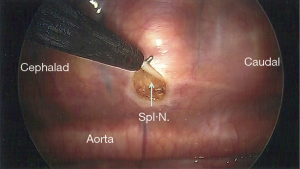
At our institution, we perform BTS. Although this has not been compared head-to-head with unilateral splanchnicectomy, we are of the opinion that pain control is better with a bilateral neurectomy. This procedure can be easily executed with a single-lumen endotracheal tube; there is no need for continuous arterial blood pressure monitoring or central venous access. We prefer a posterior approach as described by Cuschieri et al. (24). The patient is placed in the prone position with the arms abducted and flexed at the elbow. To perform a BTS, we use two 5 mm trochars. We start initially on the left side, as it has been our experience that the left pleura is often thicker with more retro pleural fat, which can make visualization of the nerves on the left side often harder than the right. Despite that, the nerves are typically easy to find if one is familiar with their normal position, a skill that is acquired after only a few operations. The first trochar is placed at the inferior apex of the scapula while the anesthetist suspends respirations. Once placed, carbon dioxide (CO2) insufflation is instilled at a pressure of 12 mmHg. A 5 mm, 30-degree angled scope is used to assess for successful trochar placement. Once the surgeon is satisfied, respirations can be resumed. In all, this initial step generally takes less than 1 minute. Next, the second trochar is placed two intercostal spaces inferior to the first and about 2 cm medially (Figure 2). It may also be placed two intercostal spaces superior to the first in the event there is elevation of the hemi-diaphragm. A third trochar may be used if needed, but this is rarely the case. The surgeon will then turn his or her attention to the posterior thorax to identify the sympathetic trunk. The arch of the aorta is used as a landmark, above which the splanchnics do not lie. The costophrenic angle is seen as well, below which the splanchnics are never found. The splanchnic nerves are seen running in an inferior and medial position from the sympathetic trunk (Figure 3). Once the splanchnics are identified, a small opening is made in the pleura on either side of the nerve with a right angle cautery. To avoid the risk of bleeding, the nerve is divided on the corpora of the vertebral body between the intercostal vessels. We recommend lifting the nerve with the right angle cautery so that division is obvious once the nerve recedes into the pleura (Figure 4). There are typically two to five nerves easily found on each side. After searching for and dividing all of the nerves, the insufflation is released, a rubber catheter is placed into the hemithorax, and large tidal volumes are given by the anesthetist. The exterior end of the rubber catheter is placed under water at the level of the skin, creating a water seal. Once the lungs are fully re-inflated, the trochars and catheter are removed, the skin incisions closed, and the procedure is repeated on the right side.
At this point, the patient is awakened and a chest X-ray is performed in the recovery room to assess for retained CO2. If the patient is stable, even in the presence of pneumothorax, observation is safe and an X-ray should be repeated on post-operative day 1. In the event the patient is unstable during or after emergence from anesthesia, urgent X-ray in the operating room (if possible, quickly) and auscultation of the chest are used to assess for tension pneumothorax. A chest thoracostomy tube is then placed on the affected side. We admit our patients for overnight observation in a non-telemetry room; however, outpatient procedures have been reported without complication. Operative time is usually less than 1 hour and the total hospital length of stay rarely exceeds 1 post-operative day.
Literature
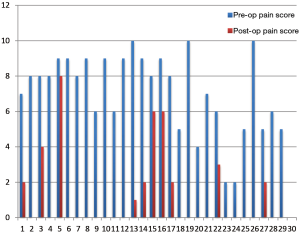
Outcomes of splanchnicectomy for palliation of pain associated with pancreas cancer are encouraging. Results of this procedure for chronic pancreatitis are more readily available in the literature but remain sparse for the treatment of malignant pancreatic disease. Pietrabissa et al. reported on 20 patients who experienced significant improvement in visual analog scores for at least 3 months post-operatively (25). In a study by Lică et al., similar outcomes on another 15 patients were demonstrated (26). At the 2010 Asian Pacific Hepato-Pancreato-Biliary meeting. Vitale et al. presented data on 36 patients who underwent BTS for pancreatic cancer. In that study, mean survival was 229 days and average pain scores dropped from 8.3 to 2.0 on a 0-10 scale. The quality of life survey on these same patients, however, only demonstrated a limited improvement. At our institution we internally reviewed the first 29 patients who underwent BTS. We too found a significant decrease in patient pain scores post-operatively (4.1 to 1.1; P value =0.004) (Figure 5).
Complications
Complications of splanchnicectomy are rare, occurring in less than 2% of patients. Similar to other thoracoscopic procedures, specific complications include pneumothorax, chylothorax, hemothorax, need for thoracotomy, persistent pain, transient hypotension, and diarrhea (3). Pneumothorax was the most commonly reported complication, as two out of the 92 patients reviewed required an unplanned thoracostomy tube.
Conclusions
Pancreatic cancer is a pervasive disease that is often incurable. As a result, pain control is a key component of palliation of this disease. Given the side effects of high-dose narcotics, interventional approaches focused on neurolysis and/or neurectomy are attractive options. This can be done using a variety of approaches, each of which has been shown to be efficacious with minimal morbidity. Currently published data is heterogeneous, and head-to-head comparisons of each is lacking. Regardless, each approach appears to be safe, effective, and technically easy to perform. There is little reason any patient with this disease should suffer from abdominal pain without an attempt at either celiac plexus block or splanchnicectomy.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Seicean A, Cainap C, Gulei I, et al. Pain palliation by endoscopic ultrasound-guided celiac plexus neurolysis in patients with unresectable pancreatic cancer. J Gastrointestin Liver Dis 2013;22:59-64. [PubMed]
- Sakorafas GH, Tsiotou AG, Sarr MG. Intraoperative celiac plexus block in the surgical palliation for unresectable pancreatic cancer. Eur J Surg Oncol 1999;25:427-31. [PubMed]
- Krishna S, Chang VT, Shoukas JA, et al. Video-assisted thoracoscopic sympathectomy-splanchnicectomy for pancreatic cancer pain. J Pain Symptom Manage 2001;22:610-6. [PubMed]
- Strong VE, Dalal KM, Malhotra VT, et al. Initial report of laparoscopic celiac plexus block for pain relief in patients with unresectable pancreatic cancer. J Am Coll Surg 2006;203:129-31. [PubMed]
- Ward EM, Rorie DK, Nauss LA, et al. The celiac ganglia in man: normal anatomic variations. Anesth Analg 1979;58:461-5. [PubMed]
- Gebhardt GF. Visceral pain mechanisms. In: Chapman CR, Foley KM, editors. Current and emerging issues in cancer pain. New York: Raven Press, 1993:99.
- Kappis M. Erfahrungen mit Lokalanästhesie bei Bauchoperationen. Verh Dtsch Gesellsch Chir 1914;43:87-9.
- Lillemoe KD, Cameron JL, Kaufman HS, et al. Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial. Ann Surg 1993;217:447-55; discussion 456-7. [PubMed]
- Sakamoto H, Kitano M, Kamata K, et al. EUS-guided broad plexus neurolysis over the superior mesenteric artery using a 25-gauge needle. Am J Gastroenterol 2010;105:2599-606. [PubMed]
- Iwata K, Yasuda I, Enya M, et al. Predictive factors for pain relief after endoscopic ultrasound-guided celiac plexus neurolysis. Dig Endosc 2011;23:140-5. [PubMed]
- Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg 1995;80:290-5. [PubMed]
- Wong GY, Schroeder DR, Carns PE, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA 2004;291:1092-9. [PubMed]
- Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. Am J Gastroenterol 2007;102:430-8. [PubMed]
- Puli SR, Reddy JB, Bechtold ML, et al. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci 2009;54:2330-7. [PubMed]
- Wyse JM, Carone M, Paquin SC, et al. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol 2011;29:3541-6. [PubMed]
- Toukhy ME, Campkin NT. Severe diarrhea following neurolytic coeliac plexus block: case report and literature review. Am J Hosp Palliat Care 2011;28:511-4. [PubMed]
- Hardy PA, Wells JC. Coeliac plexus block and cephalic spread of injectate. Ann R Coll Surg Engl 1989;71:48-9. [PubMed]
- De Conno F, Caraceni A, Aldrighetti L, et al. Paraplegia following coeliac plexus block. Pain 1993;55:383-5. [PubMed]
- Hayakawa J, Kobayashi O, Murayama H. Paraplegia after intraoperative celiac plexus block. Anesth Analg 1997;84:447-8. [PubMed]
- Loeve US, Mortensen MB. Lethal necrosis and perforation of the stomach and the aorta after multiple EUS-guided celiac plexus neurolysis procedures in a patient with chronic pancreatitis. Gastrointest Endosc 2013;77:151-2. [PubMed]
- Copping J, Willix R, Kraft R. Palliative chemical splanchnicectomy. Arch Surg 1969;98:418-20. [PubMed]
- Sadar ES, Cooperman AM. Bilateral thoracic sympathectomy--splanchnicectomy in the treatment of intractable pain due to pancreatic carcinoma. Cleve Clin Q 1974;41:185-8. [PubMed]
- Worsey J, Ferson PF, Keenan RJ, et al. Thoracoscopic pancreatic denervation for pain control in irresectable pancreatic cancer. Br J Surg 1993;80:1051-2. [PubMed]
- Cuschieri A, Shimi SM, Crosthwaite G, et al. Bilateral endoscopic splanchnicectomy through a posterior thoracoscopic approach. J R Coll Surg Edinb 1994;39:44-7. [PubMed]
- Pietrabissa A, Vistoli F, Carobbi A, et al. Thoracoscopic splanchnicectomy for pain relief in unresectable pancreatic cancer. Arch Surg 2000;135:332-5. [PubMed]
- Lică I, Jinescu G, Pavelescu C, et al. Thoracoscopic left splanchnicectomy - role in pain control in unresectable pancreatic cancer. Initial experience. Chirurgia (Bucur) 2014;109:313-7. [PubMed]