Increased risk of death due to heart disease after radiotherapy for esophageal cancer
Introduction
In the United States esophageal cancer (EC) represents 1.1% of all new cancer cases, with an estimated 18,170 new cases diagnosed in 2014 (1). Five year overall survival for this disease is poor but has improved over the last three decades. In 1975 only 4.0% of individuals diagnosed with EC survived 5 years. For individuals diagnosed in 2006, 5-year survival improved to 20.0%. This increase in survival partly reflects improvements and increased utilization of trimodality therapy [surgery, radiation therapy (RT) and chemotherapy (CT)] (2-6).
RT is an integral part of the current treatment paradigm. In a prospective, randomized trial for patients with locally advanced EC, van Hagen et al. demonstrated a doubling in median survival (24 vs. 49.4 months) with the addition of preoperative chemoradiotherapy (CRT) to esophagectomy (2). In counseling patients with EC, it is important to convey an accurate risk profile for both the short term and long term side effects of RT. Long term heart toxicity from RT has been described in both breast cancer and lymphoma, and includes pericardial disease, myocardial fibrosis, coronary artery disease (CAD), arrhythmias (including frequent persistent sinus tachycardia post-RT), and valvulopathies (7-14). Cardiac complications from treatment of EC are not as well defined and given the heart’s proximity to the esophagus, long term cardiac effects from RT are expected. With regards to short term cardiotoxic effects, imaging studies following CRT for EC have demonstrated increased myocardial perfusion abnormalities, decreased ejection fraction and pericardial effusions (15-17). These studies did not, however, correlate abnormal imaging findings with meaningful clinical outcomes, such as premature myocardial infarction or death from heart disease.
The purpose of this study was to define the long term risk of death from heart disease following RT for EC.
Materials and methods
The surveillance, epidemiology, and end results (SEER) database
The SEER Program is an authoritative source of information on cancer incidence and survival in the United States that collects data from 18 separate cancer registries representing approximately 28% of the US population. For each case submitted to the registry, important data are collected including: demographics, primary tumor site, tumor morphology and stage at diagnosis, first course of treatment, and follow-up for vital status. Data from the November 2013 SEER submission utilized for this project includes patients treated from 1973-2011 plus the Hurricane Katrina impacted Louisiana cases. Approval by an internal review board for our study was not required as all SEER database information is deidentified.
Case selection
Our study population included any patient diagnosed with EC in the database from 1973-2011. We used the SEER*Stat software (version 8.1.5) for data extraction. We identified our patient population by querying “Site recode ICD-0-3/WHO 2008” with the term “esophagus” as the primary site. For each case, we requested the following information: age, gender, year of birth, year of diagnosis, race, SEER historic stage, site specific surgery, reasons for not performing surgery, use of RT, presumed survival in months, vital status, and cause of death. Heart disease related death (HDRD) information was obtained from cause of death data extracted from the SEER database.
After extraction from the database, patients with unknown follow-up, survival less than 6 months or with unknown utilization of RT were excluded. A 6-month survival cutoff was used to exclude patients who died in short succession to, or as a result of their initial treatments. Two cohorts were then created: (I) patients who received RT as part of their initial therapy; and (II) those who did not receive RT as part of their initial therapy.
Data analysis
Pearson chi-square analyses were used to compare treatment and tumor characteristics for categorical variables. Kaplan Meier methods were then employed to analyze the primary endpoint, death from heart disease. Only death from heart disease was counted as an event in Kaplan Meier analysis. Patients were censored if they died from any other cause. Univariate and multivariate survival analyses were performed using Cox proportional-hazards regression methods. To test when heart disease specific survival (HDSS) becomes significantly different among groups, log rank test of HDSS was performed using progressive follow-up cut off points starting at 6 months from diagnosis and increasing by one-month intervals. For the purpose of data analysis, extent of surgery was defined as esophagectomy versus other. Esophagectomy was defined as either partial or total esophagectomy. Other included: no surgery, unknown if surgery performed, photodynamic therapy, electrocautery, cryosurgery, and laser ablation.
Results
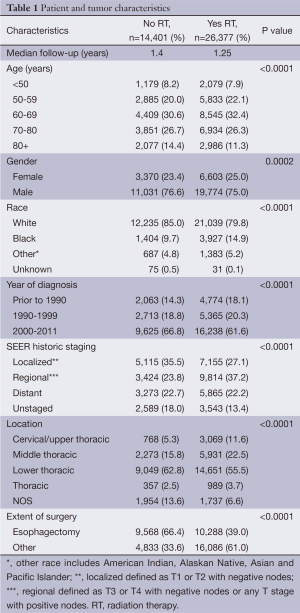
Initially, 71,595 patients were extracted from the SEER Registry with EC. After applying the exclusion criteria, 40,778 patients remained. The patient and tumor characteristics are listed in Table 1. A total of 26,377 patients received RT and 14,401 patients did not. Females, African Americans, patients diagnosed before 1990, patients with T3+ and/or node positive disease, and patients who did not undergo esophagectomy were more likely to receive RT.
Full table
All patients
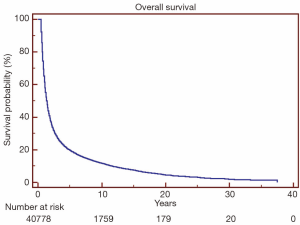
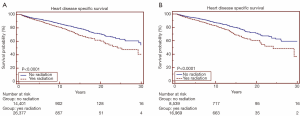
Overall survival for all patients at 5-, 10- and 20-year was 19.7%, 11.8% and 4.5%, respectively (Figure 1). HDSS analysis revealed increased risk for death from heart disease in those receiving RT as part of their initial therapy (P<0.05) (Figure 2). This survival analysis revealed an absolute risk of death from heart disease for those who received RT with their initial therapy of 2.8%, 5.3% and 9.4% at 5-, 10- and 20-year, respectively (Figure 2). Univariate analysis of both cohorts revealed that the following were found to be associated with risk of death from heart disease: RT, age, race, stage at presentation, time period of diagnosis, and known comorbid conditions keeping patients from esophagectomy (Table 2). Gender was not found to confer risk of death from heart disease. All variables found to be significant by univariate analysis were included in a multivariate analysis. RT remained predictive of death from heart disease on multivariate analysis [hazard ratio (HR) 1.46, 95% confidence interval (CI): 1.32-1.61, P<0.05]. In addition, all other variables included remained predictive of death from heart disease (Table 2). Of note, by univariate analysis, risk of HDRD in patients with known comorbid conditions was increased (HR 1.63, 95% CI: 1.24-2.14, P<0.05).
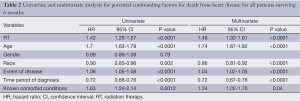
Full table
Time interval from diagnosis
Log rank test of HDSS performed at progressive monthly follow-up cut off points starting at 6 months from diagnosis revealed that the risk of HDRD became significant with a follow-up of 8 months with an absolute risk of HDRD of 0.4% (P<0.05). On multivariable analysis (including significant variables from above), risk of HDRD remained significant at 8 months (HR 1.45, 95% CI: 1.14-1.83, P<0.05).
Definitive therapy candidates
A subset analysis was performed on potential definitive therapy candidates: patients presenting with localized or regional disease. After exclusion of those with distant or unknown stages, 16,969 and 8,539 patients remained in the RT and no RT cohorts, respectively. HDSS analysis revealed increased risk for death from heart disease in those receiving RT as part of their initial therapy (P<0.05) (Figure 2). This survival analysis revealed an absolute risk of death from heart disease for those who received RT with their initial therapy 3.0%, 4.8% and 10.9% at 5-, 10- and 20-year, respectively (Figure 2). By univariate analysis, the following were found to be associated with risk of death from heart disease: RT, age, race, stage at presentation, time period of diagnosis, and known comorbid conditions keeping patients from esophagectomy (Table 3). Gender was not found to confer risk of death from heart disease. All variables found to be significant by univariate analysis were included in a multivariate analysis. RT remained predictive of death from heart disease (HR 1.62, 95% CI: 1.43-1.82, P value <0.05). In addition, all variables aside from extent of disease and known comorbid conditions remained predictive of death from heart disease (Table 3).
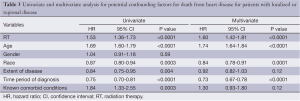
Full table
Heart disease by site of primary
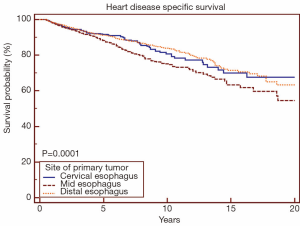
When analyzing the cohort receiving RT by site of primary tumor, mid-esophageal location was associated with increased risk of death from heart disease (Figure 3) (P<0.05). When comparing cervical/upper thoracic esophageal versus mid-esophageal sites using univariate Cox proportional-hazards regression methods, mid-esophageal site was associated with increased risk of death from heart disease (HR 1.09, 95% CI: 1.02-1.16, P<0.05). When comparing lower versus mid-esophageal sites, mid-esophageal site was again associated with increased risk of death from heart disease (HR 1.34, 95% CI: 1.17-1.53, P<0.05). There was no difference in risk of death from heart disease when comparing cervical/upper thoracic versus distal esophagus (HR 0.97, 95% CI: 0.90-1.08, P=0.76).
Discussion
The purpose of our study was to define the long term risk of death from heart disease following RT for EC. We found that for all patients receiving RT and for definitive patients receiving RT, death from heart disease occurred at 1.46 and 1.62 times the rate of those not receiving RT, respectively. To our knowledge, this is the first study to quantify risk of death from heart disease after RT for EC.
Determining the risk of death from comorbid conditions and/or treatment toxicities has become increasingly important as combined modality therapy has resulted in long-term survival for more patients (2-6). Population-based databases such as SEER have the advantage of providing large numbers of patients to lend statistical power to answer questions such as these. In addition, SEER allows for identification of patients who were not deemed surgical candidates because of medical comorbidities, which helps differentiate the effects of these negative health factors compared to the side effects of treatment. It was found that patients not undergoing surgery as a result of comorbid conditions were at higher risk of dying from heart disease (HR 1.63, P<0.05). However, when taking this into account via multivariate analysis, RT remained predictive of death from heart disease. These comorbidities may include heart disease or well-validated risk factors for heart disease including diabetes mellitus, smoking, hypertension, high cholesterol, family history and smoking (18,19). In addition, smoking (20) and other factors may potentiate the risk of RT induced cardiac toxicity.
Our analyses showed that age, race and time period of diagnosis were predictive of death from heart disease on both univariate and multivariate analylses. Time period of diagnosis was included as a variable as death from heart disease has decreased significantly over the last three decades (21,22). RT remained predictive of death from heart disease despite inclusion of these covariates in multivariate analysis. Interestingly, later disease stages also were mildly predictive of risk of death from heart disease for all patients surviving six months (HR 1.06). A potential explanation for this may be that increased burden of disease results in increased cardiac strain, leading to death from cardiovascular causes. In addition, these patients potentially received more aggressive RT, CT or surgery leading to long-term heart sequelae.
A significant increase in HDRD was detected within the first year of diagnosis for patients receiving RT, a finding that remained significant by multivariate analysis. A similar timeline was demonstrated by Darby et al., who showed a 16.3% increased relative risk for major coronary event from 0-4 years after RT for breast cancer (23). In Hodgkin’s lymphoma, studies have also shown early increases in risk of heart disease (24-26). As clinicians, this information is important as screening and treatment of other potential cardiac risk factors should take place in close interval following RT to mitigate the risk of HDRD. Further research is needed to demonstrate the most effective measures to predict and manage heart disease before and after esophageal RT.
The results of this study point to the importance of minimizing cardiac dose in RT planning. Current knowledge about the dose/volume parameters that would best limit cardiac toxicity are based on series with limited numbers of patients, on models, and on experience with other cancer types. Current cooperative group esophageal chemoradiation protocols recommend limiting the volume receiving 40 Gy to less than 50%, and the mean heart dose to less than 27 Gy (27), which is expected to limit the rate of pericarditis to less than approximately 15% (28). A volume receiving 25 Gy of less than 10% is expected to limit the rate of cardiac mortality to less than one percent based on model estimates (29). A model using retrospective data on Hodgkin’s disease and breast cancer has suggested that a uniform RT of 1/3 of the heart to 45 Gy would confer a 10% risk of long term cardiac mortality (29). In RT therapy for breast cancer, risk of HDRD has been shown to correlate with increasing dose, even in the setting of cardiac doses well below those seen in EC treatment. Darby et al. found that exposure of the heart to RT for breast cancer increased the relative rate of major coronary events by 7.4% per gray, with no apparent threshold (23). It is important to recognize that many of the patients in this study were treated before these currently understood cardiac risks and dose parameters were known. Further work is essential to further define optimal dose/volume parameters in esophageal RT.
One method of limiting cardiac dose is through intensity modulated RT (IMRT). IMRT dosimetric studies show significant decreases in dose to the heart compared to 3D conformal techniques (3DCT) when treating EC (30-32). Dosimetric analysis of patients treated with IMRT showed significant reduction in average mean heart dose (22.9 vs. 28.2 Gy) compared to theoretical four field conformal plans in one study (33). A decrease in cardiac dose may have led to decreased deaths from heart disease in a study performed by Lin et al. comparing patients who received 3DCT versus IMRT for EC (34). Cancer specific mortality was similar, but death from other causes (including cardiac-related mortality) was increased in the 3DCT cohort, leading the authors to conclude that the dosimetric advantages of IMRT may translate to clinical benefit. However, a dosimetric comparison of dose delivered to normal tissues was not completed to validate their conclusion.
Any modification of radiotherapy field size or technique done in the interest of sparing cardiac dose should be done with consideration of the well-documented risk of locoregional failure (35), which points to the importance of locoregional RT. Local control remains an important component of patient outcomes.
Patients presenting with a primary esophageal tumor in the mid-esophagus were at higher risk of death from heart disease compared to the distal esophagus, which lies adjacent to the heart. The reasons for this observation are unclear. However, cardiac doses and field sizes are often elevated for mid-esophageal tumors because of the need to extend the field posteriorly to the celiac axis, which is covered because it is hard to fully dissect with an Ivor-Lewis Esophagectomy and it represents a major risk area for lymph node metastasis for all but cervical EC (36). It is also possible that cardiac structures, such as the atria, the semilunar valves (aortic and pulmonic), and the coronary artery origins, receive higher doses of RT in patients treated for mid-esophageal primary tumors, and that damage to these structures factors into subsequent cardiovascular events.
This study provides new insight regarding long term cardiac toxicity after RT for EC. There are, however, several important limitations. First, this study is limited by the inherent, biased nature of retrospectively collected data. Second, known risk factors for heart disease (age, race, and time period of treatment) were included in our multivariate analysis to combat this bias; however, many potential risk factors for heart disease were not included in our analysis given the nature of the SEER database. Third, the SEER database does not include doses, and hence a dose response function could not be analyzed. Lastly, the SEER registry does not record information on CT, which is often used in conjunction with RT. Common chemotherapies used for EC are known to have cardiotoxic effects (37-40). It is possible that the increased risk for HDRD in patients receiving RT could reflect a combination of RT and chemo-related toxicities, and not RT-related toxicity alone.
In conclusion, while RT plays an essential role in the current treatment paradigm for EC, the use of RT in EC leads to increased risk of HDRD. Consideration of cardiac toxicity should always be done in relation to the probability of long term survival, other cardiac risk factors inherent to the patient, and the expected benefits of RT. Measures to avoid RT dose to the heart should be considered, and further work is necessary to elucidate the true risk of heart disease and the dose/volume parameters that may minimize this risk after RT for EC. The risk of HDRD becomes apparent within the first year from diagnosis. Further research is also needed to determine the most appropriate cardiac monitoring and management in the time before, during, and after definitive treatment of EC to best mitigate the risk of cardiac sequelae.
Acknowledgements
Michelle Denney BA, BS for assisting in editing and formatting.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD. Available online: http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014.
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]
- Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226-34. [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [PubMed]
- Adams MJ, Lipshultz SE, Schwartz C, et al. Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol 2003;13:346-56. [PubMed]
- Yeh ET, Tong AT, Lenihan DJ, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation 2004;109:3122-31. [PubMed]
- Boivin JF, Hutchison GB, Lubin JH, et al. Coronary artery disease mortality in patients treated for Hodgkin's disease. Cancer 1992;69:1241-7. [PubMed]
- Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA 1993;270:1949-55. [PubMed]
- Heidenreich PA, Schnittger I, Strauss HW, et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkin's disease. J Clin Oncol 2007;25:43-9. [PubMed]
- Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009;339:b4606. [PubMed]
- Henson KE, McGale P, Taylor C, et al. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer 2013;108:179-82. [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [PubMed]
- Gayed I, Gohar S, Liao Z, et al. The clinical implications of myocardial perfusion abnormalities in patients with esophageal or lung cancer after chemoradiation therapy. Int J Cardiovasc Imaging 2009;25:487-95. [PubMed]
- Tripp P, Malhotra HK, Javle M, et al. Cardiac function after chemoradiation for esophageal cancer: comparison of heart dose-volume histogram parameters to multiple gated acquisition scan changes. Dis Esophagus 2005;18:400-5. [PubMed]
- Wei X, Liu HH, Tucker SL, et al. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys 2008;70:707-14. [PubMed]
- Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837-47. [PubMed]
- Ridker PM, Buring JE, Rifai N, et al. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA 2007;297:611-9. [PubMed]
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007;99:365-75. [PubMed]
- Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2007;115:e69-171. [PubMed]
- Morbidity and mortality: 2012 chart book on cardiovascular, lung, and blood diseases. Bethesda, MD: National Heart, Lung, and Blood Institute, 2012. Available online: http://www.nhlbi.nih.gov/resources/docs/cht-book.htm
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [PubMed]
- Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al. Long-term cause-specific mortality of patients treated for Hodgkin's disease. J Clin Oncol 2003;21:3431-9. [PubMed]
- Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA 1993;270:1949-55. [PubMed]
- Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst 2007;99:206-14. [PubMed]
- Safran H, Hong TS, Haddock M, et al. RTOG 1010. A phase III trial evaluating the addition of trastuzumab to trimodality treatment of HER2-overexpressing esophageal adenocarcinoma. Radiation Therapy Oncology Group, 2013.
- Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010;76:S10-9. [PubMed]
- Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 2010;76:S77-85. [PubMed]
- Wang D, Yang Y, Zhu J, et al. 3D-conformal RT, fixed-field IMRT and RapidArc, which one is better for esophageal carcinoma treated with elective nodal irradiation. Technol Cancer Res Treat 2011;10:487-94. [PubMed]
- La TH, Minn AY, Su Z, et al. Multimodality treatment with intensity modulated radiation therapy for esophageal cancer. Dis Esophagus. 2010;23:300-8. [PubMed]
- Chen YJ, Liu A, Han C, et al. Helical tomotherapy for radiotherapy in esophageal cancer: a preferred plan with better conformal target coverage and more homogeneous dose distribution. Med Dosim 2007;32:166-71. [PubMed]
- Kole TP, Aghayere O, Kwah J, et al. Comparison of heart and coronary artery doses associated with intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys 2012;83:1580-6. [PubMed]
- Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012;84:1078-85. [PubMed]
- Oppedijk V, van der Gaast A, van Lanschot JJ, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol 2014;32:385-91. [PubMed]
- Akiyama H, Tsurumaru M, Kawamura T, et al. Principles of surgical treatment for carcinoma of the esophagus: analysis of lymph node involvement. Ann Surg 1981;194:438-46. [PubMed]
- Saif MW, Shah MM, Shah AR. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf 2009;8:191-202. [PubMed]
- Saif MW, Tomita M, Ledbetter L, et al. Capecitabine-related cardiotoxicity: recognition and management. J Support Oncol 2008;6:41-8. [PubMed]
- Arbuck SG, Strauss H, Rowinsky E, et al. A reassessment of cardiac toxicity associated with Taxol. J Natl Cancer Inst Monogr 1993;117-30. [PubMed]
- Tomirotti M, Riundi R, Pulici S, et al. Ischemic cardiopathy from cis-diamminedichloroplatinum (CDDP). Tumori 1984;70:235-6. [PubMed]