Outcomes of resected pancreatic cancer in patients age ≥70
Introduction
Pancreatic cancer remains the fourth leading cause of cancer-associated deaths in the United States (1,2). Despite advancements in multi-modality therapy pancreatic cancer remains extraordinarily lethal with a 5-year overall survival (OS) of approximately 5% (1,3). Furthermore in the United States the incidence of pancreatic cancer has continued to increase since the 1930s (4). There are greater than 43,000 cases diagnosed annually in the United States, with a large proportion dying of their disease (5).
The current accepted standard of care for resectable pancreatic cancer remains resection followed by adjuvant therapy consisting of chemotherapy. The use of post-operative radiotherapy (PORT) continues to be a topic of controversy (6). Several studies have shown an increase in OS compared to surgery alone (7-9), whereas others have shown no benefit (10-12).
In the United States the elderly population has continued to grow with a 30% increase from 2000 to 2010 (13). Additionally, the average life span has increased secondary to advancements in public health, nutrition, early detection of diseases, and continued medical progress. This increase in average life expectancy as well as advancements in cancer screening has led to a growing number of cancer diagnoses in the elderly (14).
Pancreatic cancer tends to occur at an older age, with relatively rare occurrence before the age of 45 and a sharp increase in its incidence thereafter (4). Incidence of the disease increases with advancing age, with an incidence of 29 per 100,000 in patients aged 60-64 and 91 per 100,000 in patients aged 80-84 years (15). In the United States the median age for patients diagnosed with pancreatic cancer is 72 (16). Increasing age is a well-known risk factor for the development of pancreatic cancer (17,18). In fact, approximately two-thirds of cases are diagnosed in patients greater than 65 years old (4,15). As such, more elderly patients are being diagnosed with pancreatic cancer and being considered for multi-disciplinary treatment (19). However, elderly cancer patients remain underrepresented in many clinical studies, with age greater than 70 years as a frequent exclusion criterion (20,21). As such the question remains as to whether these data can be extrapolated to the elderly population. The aim of this study was to determine the outcomes of age ≥70 patients with resected pancreatic cancer at our institution.
Materials and methods
Patients
An analysis of pancreatic cancer patients ≥70 years who underwent upfront surgical resection for pancreatic carcinoma from 2000 to 2012 was conducted to determine outcomes. Patients were excluded if they had M1 disease, lack of surgical resection, use of neoadjuvant therapy, or age <70, and unusual histologies including lymphoma, cystadenoma, intraductal palpillary mucinous neoplasm, signet ring cell carcinoma, neuroendocrine tumors, islet cell tumors such as gastrinoma, insulinoma, glucagonoma and VIPoma.
Treatment
Surgery
Patients with pancreatic head tumors underwent pancreaticoduodenectomy with or without a pylorus-sparing procedure. A minority of patients with pancreatic body or tail tumors underwent pancreaticoduodenectomy, complete pancreatectomy, or partial pancreatectomy with or without splenectomy, and/or vein resection/repair depending on the size and location of the tumor with respect to regional organs and vasculature.
Adjuvant therapy
Following surgery, patients received chemoradiation with or without neoadjuvant or adjuvant chemotherapy, chemotherapy alone, or no adjuvant therapy. Adjuvant therapy was initiated within 4 months from the time of surgery in all cases.
Patients treated with chemotherapy alone received single-agent gemcitabine. Patients treated with chemotherapy followed by radiation were treated in a similar fashion to the radiation therapy oncology group (RTOG) 9,704 protocol with 1 month of gemcitabine followed by concurrent chemoradiation with continuous infusion 5-FU or gemcitabine, followed by adjuvant gemcitabine. Patients treated with chemoradiation alone received concurrent radiation with 5-FU or gemcitabine. The median radiation dose was 50 Gy (range, 43.2-63 Gy) in 180 to 200 cGy daily fractions for a median of 28 fractions (range, 24-35 fractions) to the pancreatic tumor bed and regional lymphatics; a minority of patients received a boost to the tumor bed (median 0 Gy; range, 0-14.4 Gy).
Statistical analysis
The primary endpoint was OS, defined as the interval from surgery to date of death. Statistical analysis was performed using SPSS® version 21.0 (IBM®, Chicago, IL, USA). Progression-free survival (PFS) was also analyzed and defined as the interval from surgery to first recurrence or death. Continuous variables were compared using both Wilcoxon rank sum test and the Kruskal Wallis test as appropriate. Pearson’s Chi-square test was used to compare categorical variables. Actuarial rates of OS were calculated using the Kaplan-Meier method and the log-rank test. A Cox multivariate model was performed for OS, including all clinical, histopathologic, and treatment variables. Continuous variables for inclusion in the multivariate model were split at clinically meaningful cut-points; post-operative CA19-9 level was split at <90 and ≥90. All statistical tests were two-sided and an α (type I) error <0.05 was considered statistically significant.
Results
Patient characteristics are shown in Table 1. A total of 112 patients age ≥70 who underwent upfront pancreatic resection were analyzed with a median follow-up of surviving patients of 36 months. The median patient age was 77 years and the majority of patients presented with advanced disease and received adjuvant treatment.
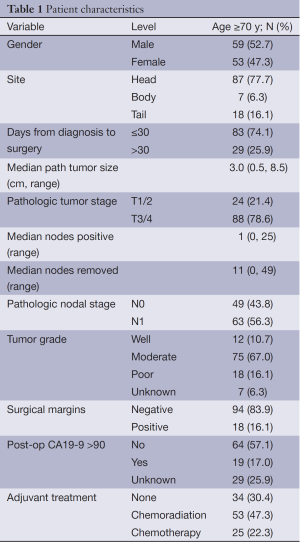
Full table
Postoperative complications are presented in Table 2. The most common complications were pancreatic leak (14.3%) and wound infection (12.5%). Postoperative 30, 60, and 90 day mortality was 2.7%, 3.6%, and 4.5%.
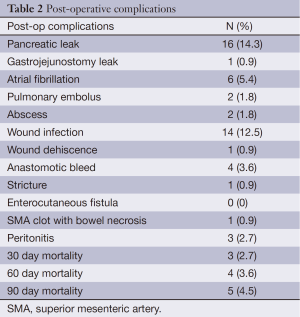
Full table
Figure 1 shows the OS and PFS Kaplan Meier curves for the patients included in this analysis. The median, 3 and 5 year OS was 20.5 months, 36%, and 19% respectively (Figure 1A). The median, 3 and 5 year PFS was 14.6 months, 24%, and 17% respectively (Figure 1B).
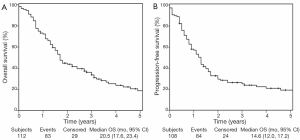
Table 3 illustrates the univariate analysis (UVA) and multivariate analysis (MVA) for OS. On UVA, increased mortality was associated with N1 status [hazard ratio (HR) 1.64: 1.05-2.56; P=0.03], post-operative CA19-9 >90 (HR 2.78: 1.56-4.93; P<0.001). There was a trend towards decreased mortality associated with adjuvant treatment with chemoradiation (HR 0.64: 0.39-1.05; P=0.08). On MVA, increased mortality was associated with N1 status (HR 1.91: 1.19-3.07; P=0.008) and postop CA19-9 >90 (HR 2.68: 1.45-4.94; P=0.002), while decreased mortality was significantly associated with adjuvant chemoradiation (HR 0.5: 0.26-0.95; P=0.04). Interestingly, there was no correlation associated with adjuvant chemotherapy alone. Age, tumor stage, interval from diagnosis to surgery, margin status, tumor site, and gender were not prognositic on UVA or MVA.
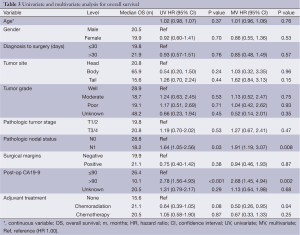
Full table
Discussion
This is one of the first studies to document outcomes and prognostic factors in patients ≥70 with pancreatic cancer treated with upfront resection with or without adjuvant therapy. Interestingly, adjuvant chemoradiation was associated with decreased mortality on MVA, whereas adjuvant chemotherapy was not prognostic. On both UVA and MVA, patients with N1 disease and post-operative CA19-9 >90 were prognostic for increased mortality.
The elderly population continues to remain underrepresented in clinical literature, representing only 25-30% of study participants (20). Secondary to this dearth of data there has been recent interest in defining the roles of different therapies in the elderly with pancreatic cancer. A retrospective study by Sehgal et al. (n=16,694) reported the rates of chemotherapy delivered and associated survival in different age groups in all patients with pancreatic cancer from the Cancer Information Resource files registry (4). They found that elderly patients with pancreatic cancer receive treatment less frequently than younger patients. Additionally, median OS was significantly less in the age >70 group (4.21 vs. 7.07 months and 7.89 months for age >70, 51-70, and ≤50 years respectively), however these patients were shown to have a comparable or better survival benefit from chemotherapy. In their UVA, age >70 was not prognostic for OS. This study also showed an OS benefit in all patients treated with radiotherapy (HR 0.47, P<0.001). Our results are in general agreement with this study, suggesting that elderly patients with pancreatic cancer do derive a benefit from treatment, specifically chemoradiotherapy (CRT).
There continues to be controversy regarding the role of PORT in resected pancreatic cancer patients (6). Several trials have shown benefit from the used of PORT in pancreatic cancer. In Gastrointestinal Tumor Study Group (GITSG) 9,173 (n=43) patients who had undergone curative resection were randomized to observation or CRT with 40 Gy split course radiation and concurrent 5-fluorouracil (5-FU) chemotherapy (9). The median survival in the CRT arm was significantly improved compared to the observation arm (20 vs. 11 months, P=0.035). Additionally, the 2-year survival rates were significantly improved with CRT vs. the observation group (42% vs. 15%; P=0.035). This initial study has led to adjuvant CRT being adopted in the United States. The European Organisation for Research and Treatment of Cancer (EORTC)-40,891 (n=218) phase III study sought to confirm these results and as such randomized patients with resected pancreatic cancer or periampullary cancer to observation or 5-FU based CRT (12). The initial data showed no difference in median survival between the two groups, (19 vs. 24.5 months; P=0.208). However, further subgroup analysis of just pancreatic tumor showed use of adjuvant CRT improved 2-year OS (23% vs. 37%; P=0.049) (22).
While these studies support the use of PORT in the treatment of pancreatic cancer there are additional data that do not support its use. The European Organisation for Research and Treatment of Cancer (ESPAC)-1 trial (n=541) compared observation, chemotherapy alone or CRT (11). They reported that adjuvant CRT worsened the median survival compared to those who did not receive CRT (16 vs. 18 months) as well as reported an inferior 2-year survival (29% vs. 49%; P=0.05). However, this study has been widely criticized for lack of quality assurance and the split-course treatment techniques. The study allowed radiation oncologists to choose their dose with a range of 40-60 Gy. Moreover, only 53% of patients enrolled in the study were included in the final analysis. Lastly the physician was able to choose how the patient was randomized and prescribe chemotherapy or “background” CRT.
While the previously mentioned trials included elderly patients, but did not specifically analyze this population, there have been two other trials that have specifically examined the elderly population. Miyamoto et al. examined pancreatic cancer patients age ≥75 (n=42) treated with CRT as adjuvant or definitive therapy (23). Median OS for the patients that received surgery followed by CRT was 20.6 months vs. 8.6 months for CRT as definitive therapy. Importantly, they showed that in this elderly population outcomes after CRT were similar to historic controls, although many patients experienced substantial treatment-related toxicity. Another study, Horowitz et al. from Johns Hopkins analyzed 655 patients from their prospectively collected database of patients who underwent resection and 5-FU based CRT (n=313) or no adjuvant treatment (n=342) (24). They showed that the 2-year survival for elderly patients receiving adjuvant CRT was significantly greater than those who received surgery alone (49% vs. 31.6%; P=0.013); however, the 5-year survival in both groups was similar (11.7% vs. 19.8% respectively; P=0.310). Upon MVA adjuvant CRT had protective effect with respect to 2-year survival [relative risk (RR) 0.59; P=0.44].
Our study differs from the aforementioned studies in the fact that we examined patients who underwent upfront surgical resection followed by no treatment, chemotherapy, and CRT. The study by Horowitz et al. compared surgery alone to CRT, and the Miyamoto et al. study compared only CRT as an adjuvant therapy to CRT as definitive therapy. While these differences do exist it appears that our data is in general agreement that elderly patients with pancreatic cancer benefit from treatment, specifically chemoradiation in the adjuvant setting.
Our study does present several inherent limitations based on the fact that this is a retrospective analysis, a time period spanning 12 years, including that fact that patient selection may influence survival. Overall, our study suggests that elderly patients with resected pancreatic cancer benefit from therapy and specifically that adjuvant CRT, however, conclusion drawn from this analysis are hypothesis generating and not definitive.
Conclusions
Our study begins to define prognostic variables associated with OS in elderly patients, a group that continues to be underrepresented in clinical research. Our data shows an increase in OS in patients that were treated with adjuvant CRT but not chemotherapy alone.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607-20. [PubMed]
- Kanda M, Fujii T, Nagai S, et al. Pattern of lymph node metastasis spread in pancreatic cancer. Pancreas 2011;40:951-5. [PubMed]
- Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol 2009;6:699-708. [PubMed]
- Sehgal R, Alsharedi M, Larck C, et al. Pancreatic cancer survival in elderly patients treated with chemotherapy. Pancreas 2014;43:306-10. [PubMed]
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- Hoffe S, Rao N, Shridhar R. Neoadjuvant vs adjuvant therapy for resectable pancreatic cancer: the evolving role of radiation. Semin Radiat Oncol 2014;24:113-25. [PubMed]
- Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975-2005). J Clin Oncol 2008;26:3511-6. [PubMed]
- Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol 2008;26:3503-10. [PubMed]
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985;120:899-903. [PubMed]
- Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet 2001;358:1576-85. [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [PubMed]
- Van Laethem JL, Hammel P, Mornex F, et al. Adjuvant gemcitabine alone versus gemcitabine-based chemoradiotherapy after curative resection for pancreatic cancer: a randomized EORTC-40013-22012/FFCD-9203/GERCOR phase II study. J Clin Oncol 2010;28:4450-6. [PubMed]
- Thakkar JP, McCarthy BJ, Villano JL. Age-specific cancer incidence rates increase through the oldest age groups. Am J Med Sci 2014;348:65-70. [PubMed]
- Kanda M, Fujii T, Suenaga M, et al. Pancreatoduodenectomy with portal vein resection is feasible and potentially beneficial for elderly patients with pancreatic cancer. Pancreas 2014;43:951-8. [PubMed]
- Altekruse SF, Kosary CL, Krapcheco M, et al. SEER Cancer Statistics Review, 1975-2007, National Cancer Institute. Available online: http://seer.cancer.gov/csr/1975_2007/
- Ries LA, Melbert D, Krapcho M, et al. eds. SEER Cancer Statistics Review, 1975-2005, National Cancer Institute. Bethesda, MD. Available online: http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER web site, 2008..
- Shore S, Vimalachandran D, Raraty MG, et al. Cancer in the elderly: pancreatic cancer. Surg Oncol 2004;13:201-10. [PubMed]
- Balcom JH 4th, Rattner DW, Warshaw AL, et al. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg 2001;136:391-8. [PubMed]
- Cooper AB, Holmes HM, des Bordes JK, et al. Role of neoadjuvant therapy in the multimodality treatment of older patients with pancreatic cancer. J Am Coll Surg 2014;219:111-20. [PubMed]
- Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061-7. [PubMed]
- Aapro MS, Köhne CH, Cohen HJ, et al. Never too old? Age should not be a barrier to enrollment in cancer clinical trials. Oncologist 2005;10:198-204. [PubMed]
- Garofalo MC, Regine WF, Tan MT. On statistical reanalysis, the EORTC trial is a positive trial for adjuvant chemoradiation in pancreatic cancer. Ann Surg 2006;244:332-3; author reply 333. [PubMed]
- Miyamoto DT, Mamon HJ, Ryan DP, et al. Outcomes and tolerability of chemoradiation therapy for pancreatic cancer patients aged 75 years or older. Int J Radiat Oncol Biol Phys 2010;77:1171-7. [PubMed]
- Horowitz DP, Hsu CC, Wang J, et al. Adjuvant chemoradiation therapy after pancreaticoduodenectomy in elderly patients with pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys 2011;80:1391-7. [PubMed]


