Palliative oxaliplatin-based chemotherapy after exposure to oxaliplatin in the adjuvant setting for colon cancer
Introduction
Colorectal cancer (CRC) remains the third most commonly diagnosed malignancy with approximately 136,830 new diagnosed cases annually in the United States. Despite recent decline in death rates for CRC, it is still responsible for approximately 10% of all cancer-related deaths in North America (1). Prognosis is dependent on stage at presentation with five-year survival rates varying from 93% in stage I (T1-2 N0) to only 44% in stage IIIC disease (N2) (2). Although patients with early stage CRC commonly undergo potentially curative resection, disease recurrence may occur and is thought to arise from occult micrometastases that are present at the time of surgery (3). This is the premise to offer adjuvant chemotherapy for those who present with stage III or II with high-risk features (4) as it could potentially eradicate micrometastatic disease.
Despite embarking on adjuvant chemotherapy, approximately 30-35% of the patients with stage III CRC eventually relapse (5-9). Although some patients may have either an isolated metastases or a local recurrence that is curable via surgery (10-12), most patients with metastatic CRC (mCRC) are incurable. The treatment in this setting generally consists of palliative chemotherapy with the goal of prolonging overall survival (OS) and maintaining quality of life. The median OS for patients with unresectable mCRC who receive best supportive care alone is approximately five to six months (13) while patients on chemotherapy in the modern era routinely live longer than two years.
Treatment for mCRC would be exactly the same for patients previously exposed or not to adjuvant therapy if it weren’t for one reason: many of those patients with recurrent disease in the modern era were already exposed to oxaliplatin during their adjuvant therapy. Thus, the emergence of oxaliplatin resistance is increasingly encountered in the clinical setting (14). A question that remains unanswered is the role of retrying oxaliplatin-based chemotherapy for a patient who has already received it in the adjuvant setting and relapsed with metastatic disease. Specific clinical trials are not yet available to guide oncologists in the evidence-based treatment of patients previously exposed to oxaliplatin. In addition, many trials evaluating oxaliplatin-based chemotherapy in the metastatic setting were conducted when oxaliplatin was not yet approved as part of adjuvant chemotherapy (15-17).
The aim of our study was to describe clinical outcomes among patients re-exposed to oxaliplatin-based chemotherapy in the metastatic setting after having received it adjuvantly.
Methods
Characteristics of the study setting and study population
The British Columbia Cancer Agency (BCCA) is a provincial-based cancer control program that is responsible for funding and providing cancer treatment to approximately 4.5 million residents in British Columbia, Canada. The agency is comprised of five comprehensive, regional cancer centers that are distributed across different catchment areas of the province so that access to care can be distributed as equitably as possible to a diverse population, regardless of financial capabilities or geographical location. All of the centers offer a full range of quality cancer services and programs including ambulatory oncology clinics, chemotherapy suites, radiation facilities, surgical services, inpatient units, palliative care, and the opportunity to participate in major oncology clinical trials for the estimated 15,000 to 20,000 new patients who are referred annually.
Records of patients who initiated adjuvant chemotherapy with either 5-fluorouracil or capecitabine plus oxaliplatin for stage III colon cancer between 2006 and 2011 at the BCCA were reviewed. Six-hundred and fifty-nine patients were identified through the BCCA Gastrointestinal Cancers Outcomes Database. Twenty patients were excluded from the analysis due to evidence of metastatic disease prior to embarking on chemotherapy while 14 patients were excluded due to rectal primaries. The final cohort consisted of 635 patients who received adjuvant oxaliplatin-based chemotherapy. All patients who were diagnosed with metastatic disease on follow-up had their baseline characteristics, date of relapse, and subsequent treatment information abstracted to an anonymized databse and analyzed. The institutional review board of the BCCA approved this study.
Statistical analyses
OS was calculated in months from the time of palliative oxaliplatin-based chemotherapy initiation to date of death or last follow-up. Progression-free survival (PFS) was calculated in months from the time of palliative oxaliplatin-based chemotherapy initiation to date of documented progression or last follow-up. Kaplan-Meier curves for both OS and PFS were generated. The log-rank test was used to assess statistical differences among variables. All tests were two-sided where a P value of <0.05 was considered statistically significant. SPSS (version 14.0) was used to conduct the analysis.
Results
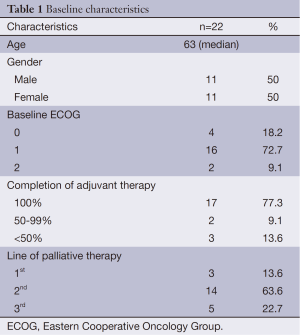
A total of 635 patients received adjuvant oxaliplatin-based chemotherapy for stage III colon cancer from 2006 to 2011. At a median follow-up of 57.9 months, 176 pts (27.7%) had recurred and 118 (18.6%) had died, 104 of them due to colon cancer. Among the 176 patients who recurred, only 22 (12.5%) were re-exposed to oxaliplatin in the metastatic setting. Median age was 63 years (range, 47-78 years) and 11 (50%) patients were male. Baseline and treatment characteristics are summarized in Table 1.
Full table
Oxaliplatin in combination with fluoropyrimidine was given as first line in 3 patients (13.6%), as second line in 14 (63.6%), and third line in 5 (22.7%). The regimens consisted of FOLFOX in 19 (86.3%) patients, FOLFOX and bevacizumab in 2 patients (9.1%), and raltitrexed plus oxaliplatin in 1 patient (4.5%). The majority of the patients (77.3%) had completed the full course of adjuvant chemotherapy. Median time from the last cycle of adjuvant oxaliplatin-based chemotherapy to the first cycle of palliative oxaliplatin-based chemotherapy was 44.3 months (range, 11.6-74.1 months). Eastern Cooperative Oncology Group (ECOG) performance status was ≤1 in 20 patients (90.9%) at the time of starting oxaliplatin. Two patients (9.1%) developed allergic reaction to oxaliplatin in the palliative setting and had it stopped before documented progression.
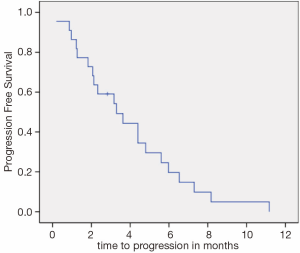
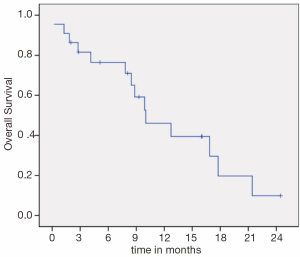
Median PFS (Figure 1) and OS (Figure 2) were 3.3 (95% CI, 1.4-5.1) and 10.0 months (95% CI, 5.3-14.6), respectively. There was no difference in PFS for patients who were re-exposed to oxaliplatin in less or more than36 months (3.6 versus 3.1 months, P=0.793, HR =0.88). Six patients (27.2%) had a PFS of more than five months.
Discussion
To the best of our knowledge, this is the largest retrospective study to date to evaluate the efficacy of oxaliplatin-based palliative chemotherapy for patients who had already received it in the adjuvant setting. The relevance of the topic is supported by the limited number of available chemotherapy options for mCRC. Our data suggest that only the minority of patients who develop metastatic disease following oxaliplatin-based adjuvant chemotherapy will eventually receive it again after disease recurrence. This may be explained by the oncologists’ belief that the disease is refractory to oxaliplatin, persistent neuropathy or simply because some patients die before embarking on subsequent chemotherapy lines. Nevertheless, almost 30% of our patients had a long-lasting PFS, suggesting some evidence of benefit for oxaliplatin re-exposure.
Potential mechanisms of platinum resistance have been proposed, including alteration in drug uptake and efflux, alteration of DNA repair mechanisms, inactivation of platinum-induced apoptosis, and resistance to DNA damage (18,19). Similar to cisplatin, oxaliplatin resistance is associated with increased excision repair cross-complementation group 1 (ERCC1) activity, which plays a role in the splicing of DNA platinum adducts. The expression levels of ERCC1, a nucleotide excision repair protein, have been correlated with response to FOLFOX therapy. In a series of 50 metastatic tumor samples from patients who had progressed on FOLFIRI therapy and ended up receiving FOLFOX, lower ERCC1 expression was associated with a median OS of 10.2 months as compared to only 1.9 months for those with increased ERCC1 expression (20). However, ERCC1 expression is not a predictive biomarker currently used for CRC patients receiving FOLFOX treatment. Further studies exploring the role of ERCC1 expression and response to FOLFOX chemotherapy are warranted.
The matter of platinum resistance has been most extensively studied in patients with ovarian cancer. The magnitude of response has been largely dependent on the duration of the platinum-free interval (21). Whether the same principle applies to CRC remains unknown, although a similar approach as that used in ovarian cancer seems reasonable. In clinical practice medical oncologists tend to divide patients who recur after oxaliplatin-based adjuvant chemotherapy in those who relapse within one year of completing adjuvant treatment and those who experience later relapses. Due to the belief that patients with a prolonged disease free interval following adjuvant oxaliplatin exposure are likely to benefit from retreatment with FOLFOX, such patients at the time of a recurrence are more likely to be offered oxaliplatin at some point in their metastatic course provided residual neuropathy is not a limiting factor. Despite insufficient statistical power, our study showed no difference in PFS for patients who were re-exposed to oxaliplatin in less or more than 36 months (3.6 versus 3.1 months, P=0.793) from time of adjuvant therapy completion. This question should be further explored in randomized trials.
Theoretically there is possible benefit of re-introduction of FOLFOX after FOLFIRI chemotherapy similar to treatment in leukemias. The theory is that oxaliplatin resistant clones would be eliminated with irinotecan-based treatment and the new clones could be susceptible to oxaliplatin re-challenge. This is similar to a phase II study that enrolled 39 irinotecan-refractory patients who had a clinical benefit after a line of cetuximab- plus irinotecan-based therapy and then a progression of disease for which underwent a new line chemotherapy and finally, after a clear new progression of disease, were retreated with the same cetuximab- plus irinotecan-based therapy (22). Overall response rate was 53.8% with a median PFS of 6.6 months. The authors concluded that rechallenging patients with the same cetuximab- plus irinotecan-based therapy may achieve a new important clinical benefit further delaying the progression of disease and improving the therapeutic options (22). This is the same concept as trying to rechallenge patients with FOLFOX after a prior exposure in the adjuvant setting.
A prior small retrospective study of patients who relapsed with metastatic disease after having received adjuvant oxaliplatin-based chemotherapy compared FOLFOX and bevacizumab versus FOLFIRI and bevacizumab in the first-line setting (23). Overall response rate was 17% in the FOLFOX/bevacizumab group (n=6) and 36% in the FOLFIRI/bevacizumab group (n=22) (P=0.22). Although this study suggests a higher response rate with FOLFIRI and bevacizumab, the small sample size limits its interpretation. However, resistance to oxaliplatin may not be complete, as shown by the occurrence of partial response in one patient at that study.
Our study does have several limitations, including the retrospective nature of the data collection. In parallel, the reasons why most of our patients did not receive oxaliplatin in the metastatic scenario could not be captured. Whether persistent neuropathy was an obstacle to re-administration of oxaliplatin is unknown. In addition, overall response rate and quality of life issues could not be determined and results were reported in terms of OS and PFS.
In conclusion, despite the fact that only a small proportion of patients who develop mCRC after adjuvant oxaliplatin-based chemotherapy receive this drug in the metastatic setting, our population-based study suggests that some patients may derive a PFS benefit. Randomized trials exploring the role of oxaliplatin re-exposure in mCRC are urgently needed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [PubMed]
- AJCC (American Joint Committee on Cancer) Cancer Staging Manual, 7th edition. Edge SB, Byrd DR, Compton CC, et al. eds. New York: Springer, 2010:143.
- Bilchik AJ, Hoon DS, Saha S, et al. Prognostic impact of micrometastases in colon cancer: interim results of a prospective multicenter trial. Ann Surg 2007;246:568-75; discussion 575-7. [PubMed]
- Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000;124:979-94. [PubMed]
- Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet 1995;345:939-44. [PubMed]
- Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696-704. [PubMed]
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109-16. [PubMed]
- Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25:2198-204. [PubMed]
- Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465-71. [PubMed]
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. [PubMed]
- Tepper JE, O’Connell M, Hollis D, et al. Analysis of surgical salvage after failure of primary therapy in rectal cancer: results from Intergroup Study 0114. J Clin Oncol 2003;21:3623-8. [PubMed]
- Goldberg RM, Fleming TR, Tangen CM, et al. Surgery for recurrent colon cancer: strategies for identifying resectable recurrence and success rates after resection. Eastern Cooperative Oncology Group, the North Central Cancer Treatment Group, and the Southwest Oncology Group. Ann Intern Med 1998;129:27-35. [PubMed]
- Scheithauer W, Rosen H, Kornek GV, et al. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ 1993;306:752-5. [PubMed]
- Kurkjian C, Murgo AJ, Kummar S. Treatment of recurrent metastatic colon cancer in the age of modern adjuvant therapy. Clin Colorectal Cancer 2008;7:321-4. [PubMed]
- Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229-37. [PubMed]
- Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol 2005;23:4866-75. [PubMed]
- Porschen R, Arkenau HT, Kubicka S, et al. Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO Colorectal Study Group. J Clin Oncol 2007;25:4217-23. [PubMed]
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307-20. [PubMed]
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 2003;22:7265-79. [PubMed]
- Shirota Y, Stoehlmacher J, Brabender J, et al. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol 2001;19:4298-304. [PubMed]
- Markman M, Markman J, Webster K, et al. Duration of response to second-line, platinum-based chemotherapy for ovarian cancer: implications for patient management and clinical trial design. J Clin Oncol 2004;22:3120-5. [PubMed]
- Santini D, Vincenzi B, Addeo R, et al. Cetuximab rechallenge in metastatic colorectal cancer patients: how to come away from acquired resistance? Ann Oncol 2012;23:2313-8. [PubMed]
- Moreau LC, Rajan R, Thirlwell MP, et al. Response to chemotherapy in metastatic colorectal cancer after exposure to oxaliplatin in the adjuvant setting. Anticancer Res 2013;33:1765-8. [PubMed]