Neoadjuvant therapy for localized pancreatic cancer: guiding principles
Introduction
Pancreatic cancer (PC) is a rising public health threat and is anticipated to account for over 48,000 cancer-related deaths by 2020—a death rate which will only be surpassed by lung cancer (1). In an era when the oncologic treatments of many solid organ cancers have made significant advances, it is sobering that the survival of patients with PC remains largely unchanged (2). Over the past 30 years, even among patients with localized PC who were managed with immediate surgery (surgery-first), the median survival rate is, at best, only 24 months (3). The majority of patients developed systemic recurrence even after margin negative (R0) resections, suggesting that PC is a systemic disease, even in the absence of radiographic evidence of distant metastases (4-6). Despite current practice guidelines, which recommend a surgery-first approach for localized PC, the application of a local therapy, such as surgery, for the treatment of a systemic disease is in contradiction with accepted oncologic principles of stage-specific treatment (7). An alternative approach is to administer early systemic therapy prior to surgery (neoadjuvant therapy) for the management of systemic disease that is suspected but not radiographically confirmed. Patients who have aggressive tumor biology and develop disease progression during neoadjuvant therapy can be spared an operative intervention with limited oncologic benefit. In this review, we will highlight the current status of PC staging, delineate recommendations for stage-specific treatment sequencing, and highlight important time points in clinical decision-making during therapy.
Limitations of current staging of PC
The foundation of modern oncology is the utilization of stage-specific therapies in order to maximize survival and quality of life for all treated patients. The success of achieving this goal is dependent on the ability to accurately discriminate between different disease stages. The staging of PC was once defined by operative exploration and the surgeon’s intraoperative assessment of resectability. However, the current staging of PC is now based on the pre-operative, objective radiologic classification of critical tumor-vessel relationships and the presence/absence of extrapancreatic disease (8). Although contrast enhanced computed tomography (CT) provides highly accurate assessments of such tumor-vessel relationships, the detection of metastatic disease is imperfect and approximately 10-20% of PC patients are discovered to have unanticipated metastases at the time of laparoscopy or laparotomy (9,10). Furthermore, over 76% of patients who undergo surgical resection will develop metastatic disease as the first evidence of disease recurrence (5,6). Therefore, the majority of patients with presumed localized PC have clinically occult metastatic disease at the time of diagnosis, and current imaging modalities cannot discriminate between patients who have microscopic metastatic disease and patients who may truly have localized disease.
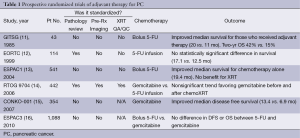
Given the high likelihood of disease recurrence after resection, multiple randomized clinical trials have assessed the benefit of adjuvant chemotherapy or chemoradiation in an effort to improve survival in patients with localized PC. Table 1 summarizes the key adjuvant studies which provide a reference to which neoadjuvant therapy must be compared. Although the trials cannot be directly compared to one another due to differences in treatment design, staging requirements, and patient characteristics, it is important to note that the median overall survival for all trials was consistently between 20-24 months (11-13,15). In addition, all trials reported a significant proportion (a minimum of 30-45%) of patients who failed to receive all intended adjuvant treatment and highlight the difficulty in administering adjuvant therapy after pancreatectomy (17). Inherent in the design of adjuvant trials is a selection bias which excludes patients who experience significant surgical morbidity or mortality from surgery. These patients do not an experience an adequate recovery to be considered for trial enrollment. When these additional patients are taken into consideration, approximately 50% of patients who undergo pancreatectomy for PC will not receive adjuvant therapy (18). Given the high risk of patients with localized PC who develop systemic disease recurrence, a reliance on adjuvant therapy to treat micrometastatic disease is troublesome when it can only be successfully administered to half of the at-risk population.
Full table
Rationale for neoadjvuant treatment sequencing
To address the limitations of adjuvant therapy, a growing interest has emerged in alternative treatment sequencing. Neoadjuvant therapy for PC has several theoretical advantages over adjuvant therapy (summarized in Table 2). In contrast to an adjuvant approach, neoadjuvant therapy ensures the delivery of all components of multimodality treatment to all patients who undergo a potentially curative pancreatectomy. Importantly, since neoadjuvant therapy offers an “induction” phase lasting approximately 2-3 months, individuals with unfavorable tumor biology who develop early metastatic disease are identified prior to surgery. Importantly, in the subset of patients (up to 20-30%) who are found to have disease progression after induction therapy (before surgery), the morbidity of an operation is avoided. When chemoradiation is utilized in neoadjuvant therapy, the delivery of chemoradiation in a well-oxygenated environment improves the efficacy of radiation and decreases the toxicity to adjacent normal tissue (19,20). The addition of radiation has important pathologic implications with several series reporting decreased rates of positive margins (R1 or R2) and node positive disease (21-23).

Full table
When neoadjuvant therapy was first introduced as an alternative to a surgery-first approach, several concerns were raised by the surgical community pertaining to safety and feasibility. Foremost was the concern that the patients with localized PC may develop local disease progression which would prevent potentially curative surgical resection; the “window of opportunity” for surgery could be lost. Over the last decade as the experience with neoadjuvant therapy has developed, concerns regarding local disease progression have not been realized. In the largest combined experience with neoadjuvant therapy for patients with resectable PC (a broad definition of resectable used in these studies), less than 1% of eligible patients were found to have isolated local disease progression at the time of re-staging after neoadjuvant therapy (before planned surgery) (24,25). Disease progression during or after neoadjuvant therapy, if it occurs, is usually seen at distant sites such as the liver, peritoneum, and lung. In addition, theoretical concerns over the toxicity of neoadjuvant therapy and the impact of treatment-related side effects on operative morbidity and mortality were also not observed (24-26). In fact, the incidence of pancreatic fistula, the most frequent serious complication associated with pancreatectomy, has been demonstrated to be reduced after neoadjuvant therapy as the treated pancreas becomes more firm with a decrease in enzyme production (21-23). With regard to overall complications, a recent analysis of the NSQIP database demonstrated no differences in 30-day mortality and postoperative morbidity rates among patients treated with neoadjuvant therapy as compared to patients who received surgery-first (27).
Importantly, the multidisciplinary care is the cornerstone of successful administration of neoadjuvant therapy. The scope of the multidisciplinary team is vast and includes medical, surgical, and radiation oncologists, diagnostic radiologists, advanced endoscopists, genetic counselors, dietitians, and endocrine specialists. Before embarking on a neoadjuvant approach, all patients should have the benefit of having their case reviewed in a multidisciplinary conference where the optimal treatment plan can be established and the course of treatment outlined prior to the initiation of any therapy. We have found that when all members of the treatment team are engaged and aligned with basic treatment principles (detailed below), the patients’ care and treatment experience are optimized.
Principle #1: radiographic determination of clinical stage of disease
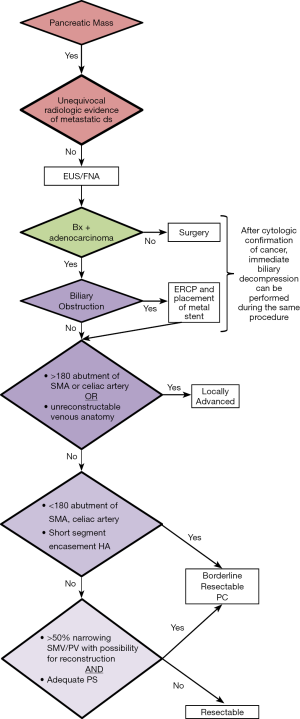
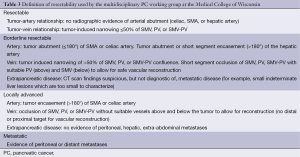
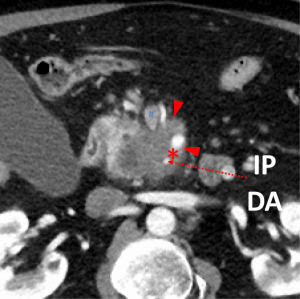
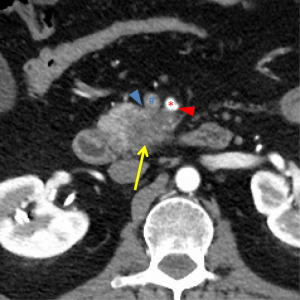
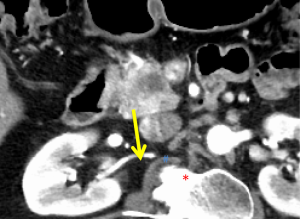
The first and most critical step in the management of PC is the determination of the clinical stage of disease and establishment of a histologic diagnosis. All disease-specific and stage-specific treatment planning is predicated on this step. With PC, it is critically important to use standardized, objective radiologic criteria for clinical staging. Modern imaging techniques have revolutionized the clinical staging of PC. Before the development of multidetector CT, up to 30% of patients with presumed resectable PC were found, at the time of operation, to have either metastatic disease or local tumor-associated vascular invasion which precluded resection (28). Currently, precise and objective anatomic radiographic criteria are used to determine the extent of the tumor-vascular relationship and to categorize clinical staging (Table 3). PC can be broadly divided into patients with inoperable disease (metastatic or locally advanced) and operable disease [borderline resectable (BLR) or resectable]. The majority of patients will present with metastatic disease, as evidenced by ascites/peritoneal implants, liver, or lung metastases. In the absence of metastatic disease, the clinical stage is determined by the relationship of the primary tumor to adjacent vasculature. As a general rule, any tumor abutment (≤180 degree tumor-vessel interface) or encasement (>180 degree) of the celiac axis, common hepatic artery, or SMA should be considered a contraindication to immediate surgery. A patient is deemed to have locally advanced, unresectable disease when: (I) the tumor encases the SMA or celiac axis, as defined by >180 degrees of the circumference of the vessel; or (II) there is occlusion of the SMPV confluence without the possibility for venous reconstruction (Figure 1). Patients who have tumor abutment, without encasement, of the SMA or celiac axis, or short segment encasement of the hepatic artery are considered to have BLR PC (Figure 2) (29). In addition, patients with tumors that cause >50% narrowing or short segment occlusion of the SMV/PV that may be amenable to reconstruction are also considered to be BLR. There is emerging consensus that even more subtle tumor-vein abutment may be best considered BLR, especially with respect to the use of neoadjuvant therapy rather than surgery-first (30). Finally, patients who have radiographic lesions which are indeterminate for metastases (usually too small to accurately characterize), even in the absence of SMA abutment or venous narrowing, are also considered by some institutions to have BLR PC (31). Radiographic findings of a resectable PC are (I) the absence of tumor-arterial abutment or encasement; and (II) <50% narrowing of the SMV/PV (Figure 3).
Full table
Our preferred algorithm for the initial diagnostic work-up and management of suspected PC is summarized in Figure 4. The single most important imaging tool for the detection and staging of PC is a CT scan. Current multi-detector protocols utilize dual-phase technique, with the acquisition of arterial phase images at 30 seconds after IV injection of contrast and portal venous images approximately 1 minute after injection. A rapid injection of intravenous contrast allows for the maximal enhancement of the pancreas and mesenteric vasculature (10). At least two phases of contrast-enhanced helical scanning are required. The first (arterial) phase is performed from the diaphragm through the horizontal portion of the duodenum in order to define the relationship of the tumor to the adjacent arteries and to determine the presence or absence of aberrant arterial anatomy. The arterial phase images are used for visualization of the primary tumor and optimal assessment of the tumor-artery relationships. Arterial phase images allow low-density adenocarcinomas to be distinguished from pancreatic neuroendocrine tumors, which are classically hypervascular in the arterial phase. The second (venous) phase is performed to define the relationship of the tumor to the surrounding venous structures (SMV, portal vein, and splenic vein) and to uncover metastases to locoregional lymph nodes and distant organs (particularly to the liver). Multidetector contrast enhanced CT provides the most comprehensive evaluation for clinical staging; we reserve additional imaging studies such as magnetic resonance imaging or positron emission testing for indeterminate lesions which are suspicious for metastatic disease.
One non-anatomic consideration which has profound implications for survival, and therefore staging, is the patient’s performance status. Especially among PC patients, striking differences in survival can be observed based on performance status alone (32-34). In a study which examined over 3,000 advanced PC patients who were treated with variety of new investigational drugs, the median survival of patients with a Karnofsky performance status (KPS) <70% was 2.4 months as compared with 5.5 months in patients with a KPS ≥70% (34). The median time to disease progression was greater in patients with a KPS score ≥70%. These findings were corroborated in the CALGB 80303 study, where PC patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0-1 experienced a median survival of 4.8-7.9 months as compared to 2.9 months in patients with an ECOG performance status of 2 (32). Because decreased performance status correlates with an increased risk of disease progression and death, performance status has been proposed as an additional criterion for BLR clinical status, even in the presence of an anatomically resectable PC (31).
Principle #2: coordination of endoscopic procedures and establishment of durable biliary drainage
Confirmation of malignancy is required in all patients prior to treatment with systemic therapy or radiotherapy. For patients with localized disease which may be amenable to surgical resection, we prefer EUS-guided FNA biopsy. The sensitivity of EUS-FNA is in range of 85% to 90% with potential false negative results of up to 15% based on tumor size and the experience of the endoscopist. False negative results can be minimized by having a cytopathologist present at the time of EUS to ensure that a cytologic diagnosis is made before the termination of the procedure. When FNA material is examined by an experienced cytopathologist, false-negative biopsies are rare, but can occur, especially when the tumors are small. Therefore, negative results from EUS-guided FNA should not be considered as proof that a malignancy does not exist, and repeat EUS-guided FNA may improve the yield of positive results in those patients with suspected malignancy. If the patient is jaundiced and EUS fails to identify a mass, an ERCP with biliary brushing may be performed followed by placement of a plastic stent (we prefer an easily removable stent when a tissue diagnosis of malignancy is not readily obtained). Importantly, high-quality CT imaging should be performed before any endoscopic intervention (EUS or ERCP) is attempted because of the risk of biopsy-induced pancreatitis, which may distort the pancreatic and peripancreatic anatomy and result in overstaging of the disease.
Although not essential for staging purposes, patients who present with jaundice will require an ERCP for biliary decompression prior to the initiation of neoadjuvant therapy. Biliary drainage and resolution of hyperbilirubinemia is required to maintain adequate liver function which is necessary for the use of several chemotherapeutic agents (25). In most cases, if on-site cytopathologic confirmation of cancer can be performed at the time of EUS, immediate ERCP can be performed with placement of a metal stent to provide more durable biliary decompression. With regards to the latter concern, large single institution experiences have demonstrated that self-expanding metal stents do not compromise future surgical resections (35). In addition, metal stents have demonstrated superior durability during neoadjuvant therapy with only a 7% rate of stent occlusion as compared to polyethylene (plastic) stents where stent occlusion has been reported in up to 45% of patients (36).
Principle #3: defining clinically important treatment responses
After accurate determination of the clinical stage, the assignment of type(s) of neoadjuvant therapy and the duration of therapy is developed with the intent to both treat radiographically occult micrometastatic disease (present in the majority of patients) and to maximize local control. Importantly, the assessment of treatment response is critically important and should be performed following the completion of any treatment modality. In patients with localized PC, defining treatment response to therapy can be particularly challenging as, by definition, measurable extrapancreatic disease does not exist. At the Medical College of Wisconsin, treatment response is assessed using three critically important criteria: (I) the presence or absence of clinical benefit (for example, the resolution of pain); (II) CT findings to suggest stable or responding disease vs. disease progression (change in cross-sectional diameter of the tumor); and (III) the decrease or increase in serum level of carbohydrate antigen 19-9 (CA19-9). Clinical benefit and CA19-9 response are used as surrogate markers of response under the assumption that extrapancreatic micrometatatic disease has likely responded to therapy if the condition of the patient improves and the level of CA19-9 declines. Although modern chemotherapy regimens such as FOLFIRINOX (5-fluorouracil, oxaliplatin, irinotecan, and leucovorin) and gemcitabine/nab-paclitaxel have been associated with 30-40% response rates among patients with more advanced disease, the majority of patients with localized PC are likely to have minimal to modest changes in tumor size (9,37-39). Moreover, although tumors may demonstrate a decrease in overall size, the relationship of the tumor to adjacent vessels generally does not change. A change in clinical stage, reflecting a change in local tumor-vessel anatomy, in response to neoadjuvant therapy has been reported to occur in less than 1% of cases (37). Therefore, the utilization of restaging imaging should primarily be performed to: (I) identify disease progression, whether it be local or distant, which would alter clinical management and; (II) facilitate operative planning. Importantly, careful attention to radiographic findings allows for a detailed preoperative plan, especially when vascular reconstruction is anticipated. It is especially important that vascular resections occur as planned events rather than an emergent response to vascular injury, as unexpected vascular injuries can ultimately compromise the completeness of the resection resulting in a positive margin (40,41).
CA19-9 has been demonstrated to be a useful prognostic marker in patients with PC. Among patients with localized PC, a decrease in CA19-9 in response to neoadjuvant therapy has previously been reported to correlate with overall survival. A greater than 50% reduction in CA19-9 levels in response to neoadjuvant therapy has been associated with an improved overall survival (42,43). Importantly, among patients who undergo neoadjuvant therapy and pancreatic resection, the normalization of CA19-9 in response to therapy has been a highly favorable prognostic factor and has been associated with a median survival of 46 months. Equally important is the recognition that an increase in CA19-9 level after therapy correlates with disease progression. Although the majority of patients will experience a decline in CA19-9 in response to neoadjuvant therapy, approximately 20% of patients will have an increase in CA19-9, and among these patients, metastatic disease was detected in 50% of cases (44). Therefore, clinicians should have a low threshold for expanding the diagnostic workup (MRI of liver or PET) prior to surgery in patients who have a rising CA19-9 after neoadjuvant therapy.
Principle #4: development of a stage-specific treatment plan
Resectable PC
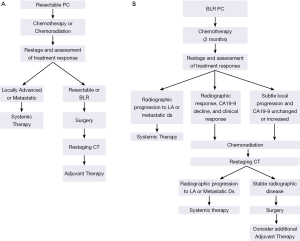
Outside of a clinical trial, neoadjuvant treatment of resectable PC may consist of chemotherapy alone or chemoradiation. If chemoradiation is used, gemcitabine combined with external-beam radiation therapy is favored (Figure 5A). This regimen is a slight modification of the neoadjuvant treatment schema reported by Evans and colleagues and includes a standard fractionation course of radiation therapy (1.8 Gy/day, M-F, 28 fractions) to a total dose of 50.4 Gy, with concurrent weekly gemcitabine given on day 1 (day −2 to +1) at a dose of 400 mg/m2 at fixed dose rate over 40 minutes (25). This program resulted in a median survival of almost 3 years in those patients who completed all therapy to include surgery (24). Restaging with pancreatic protocol CT imaging is completed 4 weeks after the last radiation treatment and in the absence of disease progression, patients are then brought to surgery. The recent reports of both FOLFIRINOX and gemcitabine/nab-paclitaxel, which demonstrated efficacy in patients with advanced disease (38,39), have generated enthusiasm for their use in patients with localized disease, especially those with BLR disease (26,45,46). Acknowledging that the use of chemoradiation remains controversial, neoadjuvant FOLFIRINOX or gemcitabine/nab-paclitaxel delivered over approximately 2 months also represents a logical treatment alternative for patients with resectable disease.
BLR PC
Patients with BLR PC are fundamentally different from those with resectable disease in that they are: (I) at higher risk for harboring radiographically occult distant metastatic disease; (II) at the highest possible risk for a positive margin of resection due to tumor-artery abutment; (III) require a more complex operation usually involving vascular resection and reconstruction, and therefore; (IV) there is a greater possibility that, despite the best efforts of the physician team, a surgical procedure may yield no oncologic benefit for the patient. For these reasons, investigators have applied a more robust level of selection consisting of a longer period of induction therapy, often including chemotherapy followed by chemoradiation prior to considering surgery. The chemoradiation portion of induction therapy has been thought to be particularly important for those patients with arterial abutment in the hope of sterilizing at least the periphery of the tumor and thereby preventing a positive margin of resection.
Our preferred off-protocol neoadjuvant treatment schema for patients with BLR PC consists of an initial two months of systemic therapy followed by chemoradiation (Figure 5B). The choice of systemic agents for initial treatment has evolved from gemcitabine-based therapies to consideration of FOLFIRINOX, GTX, gemcitabine/nab-paclitaxel, or other combination therapies (26,39,47-50). After the delivery of systemic therapy, patients are restaged with particular attention to treatment response indicators (clinical, radiographic, biochemical). Importantly, in the absence of a robust response to chemotherapy alone (and assuming no evidence of distant disease), it is our practice to proceed directly to chemoradiation (as discussed above) to minimize the risk of local disease progression after chemotherapy. Treatment sequencing in patients with BLR PC aims to both treat presumed (radiographically occult) systemic disease without the delay imposed by a surgery-first treatment approach—while also avoiding local disease progression which may sacrifice a window of opportunity for surgical resection of the primary tumor. Patients who have stable disease following two months of chemotherapy [no change on CT imaging and a modest decline (or no decline) in CA19-9] should transition to chemoradiation rather than second line systemic therapy which may increase the risk for local disease progression. As therapies evolve and therapeutic options increase, this recommendation may change. Importantly, we may be entering a new era in the management of localized PC, where small but clinically significant advances in systemic therapies improve control of distant metastases and patient survivals to the extent that more patients survive long enough to experience challenging symptoms of local-regional disease recurrence/progression for which we have little contemporary experience. The importance of local disease control, especially in patients with potentially operable disease, cannot be overstated—as clinically significant local-regional disease recurrence may be preventable with an optimal operation and the consistent delivery of multimodality therapy to include chemoradiation either before or after surgery.
Principle #5: avoid high risk operations in high risk patients
Following the completion of neoadjuvant therapy, at the time of restaging prior to surgery, it is important that a careful assessment of the patient’s performance status and medical comorbidities be re-evaluated. Several studies have demonstrated that patients with poor performance status or uncontrolled comorbidities are likely to experience postoperative morbidity and mortality (51-53). The physiologic stress associated with preoperative therapy has the potential to identify/expose patients with poor physiologic reserve who may not tolerate a large operation. If a given patient cannot tolerate induction therapy, they are unlikely to tolerate five to seven hours of surgery and recover to their pre-diagnosis level of independence with self-care. Identification of such patients at the time of diagnosis without the “stress test” of induction therapy may be difficult—a surgery-first treatment approach may incur a higher morbidity and mortality in the absence of the selection advantage afforded neoadjuvant treatment sequencing. During and after induction therapy, physicians can more accurately assess the physiologic tolerance of an individual patient to undergo major surgery. Perhaps even more importantly, after neoadjuvant therapy, the patient and their family have an improved understanding of the disease, are much better informed (than within one to two weeks following diagnosis) and evolve a much more educated opinion regarding their physicians’ recommendation for or against an operation.
In our recent experience, among older patients who completed neoadjuvant therapy but did not undergo surgery (due to either disease progression seen on restaging or a decline in performance status due to the combination of treatment toxicity and underlying comorbidities), the median overall survival was the same regardless of why surgery was not performed. A decline in performance status due to evolving medical comorbidities or the failure to recover from treatment-related toxicity was just as powerful a predictor of poor outcome as was the development of metastatic disease. This confirms previous reports of the powerful impact of performance status on response to anticancer therapy and overall survival in patients with solid tumors (54).
Conclusions
In contrast to many other solid organ tumors, treatment sequencing for patients with localized PC remains highly controversial. The limited (and clinically insignificant) gains in survival for patients with localized PC over the past three decades have been due, in part, to the current inability of physician teams to accurately stage patients. This has resulted in the overuse of surgery in patients with locally advanced and metastatic disease. In contrast to a surgery-first strategy, neoadjuvant treatment sequencing will guide the selection of patients for surgery and help to identify those patients with progressive disease for whom an operation has little oncologic benefit. Considering that surgery has a modest impact on the natural history of PC in most patients, a neoadjuvant approach to treatment sequencing is gaining support from clinicians of all specialties and will form the backbone for most future studies of multimodality therapy in localized PC.
Acknowledgements
The authors acknowledge the support of the We Care Fund for Medical Innovation and Research, the Ronald Burklund Eich Pancreatic Research Fund, and the Lockton Funds for Pancreatic Cancer Research from the Department of Surgery at the Medical College of Wisconsin. S Tsai acknowledges support from the Institutional Research Grant # 86-004-26 from the American Cancer Society. The authors would like to thank Wendy Behrs for assistance with manuscript preparation. The Pancreatic Cancer Program at MCW is grateful for the courageous patients and families who have formed the basis of our experience and reinforce the importance of multimodality care.
Funding: American Cancer Association Pilot Grant, We Care Fund for Medical Innovation and Research, Ronald Burkland Eich Pancreatic Cancer Research Fund, Advancing a Healthier Wisconsin.
Disclosure: The authors declare no conflict of interest.
References
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [PubMed]
- Stat Fact Sheet: Pancreas Cancer [10/30/2013]. Available online: http://seer.cancer.gov/statfacts/html/pancreas.html
- Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol 2012;19:169-75. [PubMed]
- Sohal DP, Walsh RM, Ramanathan RK, et al. Pancreatic adenocarcinoma: treating a systemic disease with systemic therapy. J Natl Cancer Inst 2014;106:dju011. [PubMed]
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13. [PubMed]
- Gnerlich JL, Luka SR, Deshpande AD, et al. Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch Surg 2012;147:753-60. [PubMed]
- Tempero MA, Arnoletti JP, Behrman S, et al. Pancreatic adenocarcinoma. J Natl Compr Canc Netw 2010;8:972-1017. [PubMed]
- Appel BL, Tolat P, Evans DB, et al. Current staging systems for pancreatic cancer. Cancer J 2012;18:539-49. [PubMed]
- Tran Cao HS, Balachandran A, Wang H, et al. Radiographic tumor-vein interface as a predictor of intraoperative, pathologic, and oncologic outcomes in resectable and borderline resectable pancreatic cancer. J Gastrointest Surg 2014;18:269-78; discussion 278. [PubMed]
- Raman SP, Horton KM, Fishman EK. Multimodality imaging of pancreatic cancer-computed tomography, magnetic resonance imaging, and positron emission tomography. Cancer J 2012;18:511-22. [PubMed]
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985;120:899-903. [PubMed]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776-82; discussion 782-4. [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [PubMed]
- Regine WF, Winter KW, Abrams R. RTOG 9704 a phase III study of adjuvant pre and post chemoradiation (CRT) 5-FU vs. gemcitabine (G) for resected pancreatic adenocarcinoma. J Clin Oncol 2006;24:abstr 4007.
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [PubMed]
- Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304:1073-81. [PubMed]
- Wu W, He J, Cameron JL, et al. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann Surg Oncol 2014;21:2873-81. [PubMed]
- Mayo SC, Gilson MM, Herman JM, et al. Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg 2012;214:33-45. [PubMed]
- Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg 1992;127:1335-9. [PubMed]
- Pilepich MV, Miller HH. Preoperative irradiation in carcinoma of the pancreas. Cancer 1980;46:1945-9. [PubMed]
- Takahashi H, Ogawa H, Ohigashi H, et al. Preoperative chemoradiation reduces the risk of pancreatic fistula after distal pancreatectomy for pancreatic adenocarcinoma. Surgery 2011;150:547-56. [PubMed]
- Raut CP, Evans DB, Crane CH, et al. Neoadjuvant therapy for resectable pancreatic cancer. Surg Oncol Clin N Am 2004;13:639-61. [PubMed]
- Willett CG, Lewandrowski K, Warshaw AL, et al. Resection margins in carcinoma of the head of the pancreas. Implications for radiation therapy. Ann Surg 1993;217:144-8. [PubMed]
- Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3496-502. [PubMed]
- Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3487-95. [PubMed]
- Christians KK, Tsai S, Mahmoud A, et al. Neoadjuvant FOLFIRINOX for borderline resectable pancreas cancer: a new treatment paradigm? Oncologist 2014;19:266-74. [PubMed]
- Cooper AB, Parmar AD, Riall TS, et al. Does the use of neoadjuvant therapy for pancreatic adenocarcinoma increase postoperative morbidity and mortality rates? J Gastrointest Surg 2015;19:80-6; discussion 86-7. [PubMed]
- Friess H, Kleeff J, Silva JC, et al. The role of diagnostic laparoscopy in pancreatic and periampullary malignancies. J Am Coll Surg 1998;186:675-82. [PubMed]
- Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13:1035-46. [PubMed]
- Evans DB, Farnell MB, Lillemoe KD, et al. Surgical treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol 2009;16:1736-44. [PubMed]
- Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008;206:833-46; discussion 846-8. [PubMed]
- Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 2010;28:3617-22. [PubMed]
- Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 2005;23:3509-16. [PubMed]
- Storniolo AM, Enas NH, Brown CA, et al. An investigational new drug treatment program for patients with gemcitabine: results for over 3000 patients with pancreatic carcinoma. Cancer 1999;85:1261-8. [PubMed]
- Mullen JT, Lee JH, Gomez HF, et al. Pancreaticoduodenectomy after placement of endobiliary metal stents. J Gastrointest Surg 2005;9:1094-104; discussion 1104-5. [PubMed]
- Aadam AA, Evans DB, Khan A, et al. Efficacy and safety of self-expandable metal stents for biliary decompression in patients receiving neoadjuvant therapy for pancreatic cancer: a prospective study. Gastrointest Endosc 2012;76:67-75. [PubMed]
- Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012;118:5749-56. [PubMed]
- Von Hoff DD, Goldstein D, Renschler MF. Albumin-bound paclitaxel plus gemcitabine in pancreatic cancer. N Engl J Med 2014;370:479-80. [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [PubMed]
- Ravikumar R, Sabin C, Abu Hilal M, et al. Portal vein resection in borderline resectable pancreatic cancer: a United Kingdom multicenter study. J Am Coll Surg 2014;218:401-11. [PubMed]
- Smoot RL, Christein JD, Farnell MB. Durability of portal venous reconstruction following resection during pancreaticoduodenectomy. J Gastrointest Surg 2006;10:1371-5. [PubMed]
- Boone BA, Steve J, Zenati MS, et al. Serum CA 19-9 response to neoadjuvant therapy is associated with outcome in pancreatic adenocarcinoma. Ann Surg Oncol 2014;21:4351-8. [PubMed]
- Katz MH, Varadhachary GR, Fleming JB, et al. Serum CA 19-9 as a marker of resectability and survival in patients with potentially resectable pancreatic cancer treated with neoadjuvant chemoradiation. Ann Surg Oncol 2010;17:1794-801. [PubMed]
- Aldakkak M, Christians, KK, et al. Pre-Treatmet CA 19-9 Does Not Predict the Response to Neoadjuvat Therapy in Patients with Localized Pancreatic Cancer. HPB 2015. Available online: http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1477-2574
- Mahaseth H, Brutcher E, Kauh J, et al. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas 2013;42:1311-5. [PubMed]
- Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12-7. [PubMed]
- Fine RL, Fogelman DR, Schreibman SM, et al. The gemcitabine, docetaxel, and capecitabine (GTX) regimen for metastatic pancreatic cancer: a retrospective analysis. Cancer Chemother Pharmacol 2008;61:167-75. [PubMed]
- Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 2011;29:4548-54. [PubMed]
- Kim EJ, Ben-Josef E, Herman JM, et al. A multi-institutional phase 2 study of neoadjuvant gemcitabine and oxaliplatin with radiation therapy in patients with pancreatic cancer. Cancer 2013;119:2692-700. [PubMed]
- Pilgrim CH, Tsai S, Tolat P, et al. Optimal management of the splenic vein at the time of venous resection for pancreatic cancer: importance of the inferior mesenteric vein. J Gastrointest Surg 2014;18:917-21. [PubMed]
- Cohen ME, Bilimoria KY, Ko CY, et al. Effect of subjective preoperative variables on risk-adjusted assessment of hospital morbidity and mortality. Ann Surg 2009;249:682-9. [PubMed]
- Scarborough JE, Bennett KM, Englum BR, et al. The impact of functional dependency on outcomes after complex general and vascular surgery. Ann Surg 2015;261:432-7. [PubMed]
- Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg 2009;250:449-55. [PubMed]
- Miura JT, Krepline AN, George B, et al. Treatment Sequencing in Patients with Pancreatic Cancer: The Use of Neoadjuvant Therapy in Those 75 Years of Age and Older. Surgery 2015. In Press.