Establishing the link between hepatitis B virus infection and colorectal adenoma
Introduction
Viruses and their relationship to malignancy is well understood, and has been established in those that chronically affect liver including hepatitis B virus (HBV) and hepatitis C virus (HCV) (1-3). Carcinogenesis in most cases is theorized to occur secondary to critical viral genes interfering with host genes, resulting in the activation of cellular proto-oncogenes and/or the inactivation of anti-oncogenes with their products subsequently leading to neoplastic processes.
Hepatitis B is a formidable health problem worldwide, with an estimated 1 million people dying annually from hepatitis B and its complications. About 90% of healthy adults will recover from acute hepatitis B infection; however, 5-10% worldwide (400 million people) still are infected with chronic hepatitis B (CHB) infection (4). HBV is a small enveloped, partially double-stranded DNA virus from the hepadna family, which integrates its DNA into the host genome (5). The proposed carcinogenic mechanism of HBV occurs through its direct integration of DNA into a host genome, causing production of transforming proteins, mainly protein X and pre S-S proteins (6-9). The progression to hepatocellular carcinoma occurs through chronic inflammation and repeated cellular regeneration of hepatocytes leading to cirrhosis and then malignancy, typically occurring after 25-30 years of infection (10). Chronic HBV infection has also been linked to other malignancies including pancreatic cancer, and hematological malignancies such as non-Hodgkin’s lymphoma and chronic lymphocytic leukemia (11-13).
Colorectal adenocarcinoma is the third most common cancer and the third leading cause of cancer-related deaths in both men and woman. In 2011, there were 141,210 new cases of colorectal cancer and 49,380 colorectal cancer-related deaths (14). American Cancer Society recently found the rate people are diagnosed with colorectal cancer in the US has dropped 30% in the last 10 years for those 50 years and older, which is credited to more people following the recommended screening guidelines. Establishing a link with early detection of colorectal adenomas in chronically infected HBV patients can potentially prevent future development of CRC. Recently Rustagi et al. suggested a link between chronic hepatitis C (CHC) and higher incidence of colorectal adenoma occurring through a T cell mediated process (15). They proposed the mechanism via T cells mediated cascade of unopposed B cell induction initiated by the HCV envelope protein E2 binding to CD81 molecule on B cells, lowering the threshold for B cell proliferation (16). In addition, they suggested the HCV core protein may also function as a gene regulator, with the ability to inhibit the tumor suppressor gene p53 and induce the growth factor NF-κB (17). To our knowledge, a link between chronic HBV and colorectal adenomas has yet to be explored. We aimed to identify the incidence of colorectal adenomas in HBV patients and whether chronic HBV infection is an independent risk factor for colorectal adenomas.
Methods
Patients
Institutional Review Board (IRB) approval was obtained from Nassau University Medical Center in East Meadow, New York. This is a 1,200-bed tertiary care teaching hospital in East Meadow, New York affiliated with North Shore Long Island Jewish Health System. A retrospective chart review was performed between July 1, 2009 to March 21, 2011. We established a database of 558 consecutive patients undergoing screening or diagnostic colonoscopy that had previously been screened or diagnosed with hepatitis B. Data were collected including age, sex, race, smoking, alcohol, aspartate aminotransferase (AST), alanine aminotransferase (ALT), hypertension, diabetes mellitus, and dyslipidemia. Patients with incomplete chart records or with colon cancer, inflammatory bowel disease, incomplete colonoscopies were excluded from our study. Information regarding whether the colonoscopy was screening or diagnostic was based on United States Preventative Services Task Force (USPSTF) guidelines. We recorded colonoscopy findings included presence of adenoma, adenoma >9 mm, two or more adenomas. In addition we also distinguished adenoma depending on location whether proximal/distal colon, size, largest adenoma location.
Statistical analysis
Comparisons between categorical variables were made using a Chi Square test and a t-test was used for comparisons between continuous variables. Unconditional logistic regression was used to generate age-, gender-and race-adjusted odds ratios and their 95% confidence intervals (CI) comparing hepatitis B group with control. Statistical analyses were performed with SAS 9.3 software.
Results
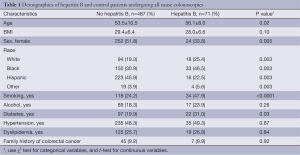
When examining patient demographics in Table 1, there were a total of 487 individuals in the control group vs. 71 in the CHB group. There was a significant age difference between HBV group and control group (P<0.05) with HBV infected patients significantly older 53.5±10.6 vs. 56.1±8.0, P=0.048. In addition, there was a higher occurrence of HBV in blacks compared to whites, Hispanics, and others (46%, 25%, 22%) respectively. Females were less likely to have hepatitis B at 51.8% compare to 33% with hepatitis B (P=0.005). Smoking was more prevalent in HBV group compared to control group (P=0.0001). There was no significant difference between hypertension, dyslipidemia or family history of colon cancer, however there was a significantly increased rate of diabetes 31.0% vs. 19.9% seen in the hepatitis B group (P=0.03). Similarly, there was no significant difference in alcohol consumption between HBV group and control group. There was significant difference in BMI between control and HBV group (28.8±7.5 vs. 26.4±9.1, P=0.02).
Full table
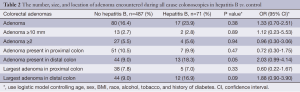
Table 2 shows the number, size, and location of adenoma encountered during either screening or diagnostic colonoscopy after adjusting for age, sex, race, BMI, diabetes, smoking, and alcohol use. There was a higher incidence of adenoma presence found in the HBV group compared to control (23.9% vs. 16.4%), however this did not reach statistical significance (P=0.38). There was a statistically significant higher number of adenomas present in the distal colon compared to control (OR =2.16; 95% CI, 1.06-4.43; P=0.04). We found no association with the presence of colorectal adenomas in CHB patients with regard to multiplicity, size, or presence in the proximal colon. There was a significantly higher number of HBV patients with elevated AST and ALT compare to the control group (59% vs. 35%, P<0.0001).
Full table
Discussion
This article presents one of the first studies looking for an association between hepatitis B and colorectal adenomas. Our data shows evidence for an association between HBV infected individuals and a higher detected rate of adenomas located in the distal colon. The carcinogenic properties of HBV infection is likely multi-factorial and remains a heavily studied area of interest. One hypothesis that is suggested which highlights the carcinogenesis process is through the chromosomal segments containing P53, PB Wnt/β-catenin, transforming growth factor β (TGF-β) and Ras signaling pathways. HBV X protein (HBx) can bind to the p53 tumor-suppressor protein, interfering with the role p53 plays in the cellular response to repair DNA damage. The role of p53 as a key player in the colorectal tumor progression has previously been demonstrated in the adenoma-carcinoma time sequence occurring in the transformation of colorectal adenomas into carcinomas (18), and may serve as a link between HBV and colorectal cancer. Hepatitis B presents with extra-hepatic manifestations including polyarteritis nodosa and glomerular disease, which are thought to be mediated by circulating immune complexes, occurring in ~10% to 20% of patients with chronic HBV infection (19-22). Fahal et al. looked at the detection rate of hepatitis B surface antigen (HBsAg) and gastrointestinal tract tumors. The data did not find a significant relationship although they were able to detect HBsAg in primary hepatocellular (25%), gastric (12%), rectal (10%) and colonic carcinoma (8%) (23). Previously other viruses (CMV, HPV, and EBV) were investigated by Boguszaková et al. although no viral antigen was detected in 13 patients with adenocarcinoma of colon and 10 patients with endoscopic polypectomies for colon adenoma using a DNA probe (24). Future studies are warranted looking specifically at HBV and mechanisms inducing carcinogenesis, specifically colorectal adenocarcinoma.
African Americans currently have 20% higher incidence of colon cancer when compared to whites (25). Whether this is genetic, environmental, ability to access health care, or a combination is unknown. In 2010, African-Americans had the highest rate of acute or recent HBV infection, at 1.7 cases per 100,000 persons, 4-fold higher than the rate for whites (26). Similarly, our findings show the highest percentage of the HBV population was African American compared to whites or other groups. It would be of potential interest for future studies to examine if African Americans with HBV have a higher incidence of adenoma compared to the other races with HBV infection through a different mechanism of genetic susceptibility. In addition, other independent risk factors have been identified that increases the risk for CRC such as smoking, metabolic syndrome, increasing age and family history of colorectal cancer to name many of the few (27). Based on evidence identifying African Americans as having a higher colorectal cancer incidence and mortality, a younger mean age of diagnosis, and evidence for more proximal colonic cancer distribution in comparison to whites (28), the American College of Gastroenterology recommends earlier screening at age 45 instead of age 50 recommended for other races (29). This demonstrates the necessity of large scale studies to delineate higher risk patient populations such as ones with HBV infection and the importance of implementing new guidelines for screening and prevention. Given that African American population have a higher incidence of HBV infection and are at higher risk of colorectal adenomas, future large scale studies are recommended for this population.
Strengths of this article include adding data to a topic that is currently limited and unavailable. We hope through our study to encourage further interest for future large scale, prospectively collected data that can answer many of the questions created from our study, and can promote campaigns for HBV vaccination and HBV infection prevention, ultimately decreasing the risk of CRC especially in high risk populations. Limitations include a retrospective design, small sample size, and unknown length of HBV diagnosis. Additionally, the HBV groups were not analyzed in regards to their viral load or genotype. Despite these limitations HBV remains a difficult disease to treat and many of the patients face a chronic disease, therefore risk factors for other malignancies and complications should be understood to further advance longevity of life in these patients.
Conclusions
In conclusion, we have demonstrated that there is a statistically significant association between CHB infection and the presence of colorectal adenomas in the distal colon. Future large scale prospective studies are needed with specific focus on duration of disease, viral load, and genotype are needed to further delineate our findings.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Simonetti RG, Cammà C, Fiorello F, et al. Hepatitis C virus infection as a risk factor for hepatocellular carcinoma in patients with cirrhosis. A case-control study. Ann Intern Med 1992;116:97-102. [PubMed]
- Kalaitzakis E, Gunnarsdottir SA, Josefsson A, et al. Increased risk for malignant neoplasms among patients with cirrhosis. Clin Gastroenterol Hepatol 2011;9:168-74. [PubMed]
- Fattovich G, Pantalena M, Zagni I, et al. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol 2002;97:2886-95. [PubMed]
- Hepatitis B Foundation. Hepatitis B Statistics. 2014. Accessed on May 25th, 2014. Available online: http://www.hepb.org/hepb/statistics.htm
- Lee WM. Hepatitis B virus infection. N Engl J Med 1997;337:1733-45. [PubMed]
- Samal J, Kandpal M, Vivekanandan P. Molecular mechanisms underlying occult hepatitis B virus infection. Clin Microbiol Rev 2012;25:142-63. [PubMed]
- Matsuoka S, Nirei K, Tamura A, et al. Influence of occult hepatitis B virus coinfection on the incidence of fibrosis and hepatocellular carcinoma in chronic hepatitis C. Intervirology 2008;51:352-61. [PubMed]
- Squadrito G, Pollicino T, Cacciola I, et al. Occult hepatitis B virus infection is associated with the development of hepatocellular carcinoma in chronic hepatitis C patients. Cancer 2006;106:1326-30. [PubMed]
- Pollicino T, Squadrito G, Cerenzia G, et al. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology 2004;126:102-10. [PubMed]
- Arababadi MK, Nasiri Ahmadabadi B, Kennedy D. Current information on the immunologic status of occult hepatitis B infection. Transfusion 2012;52:1819-26. [PubMed]
- Wang Y, Yang S, Song F, et al. Hepatitis B virus status and the risk of pancreatic cancer: a meta-analysis. Eur J Cancer Prev 2013;22:328-34. [PubMed]
- Liu WP, Zheng W, Wang XP, et al. An analysis of hepatitis B virus infection rate in 405 cases of non-Hodgkin lymphoma. Zhonghua Xue Ye Xue Za Zhi 2011;32:521-4. [PubMed]
- Rossi D, Sala L, Minisini R, et al. Occult hepatitis B virus infection of peripheral blood mononuclear cells among treatment-naive patients with chronic lymphocytic leukemia. Leuk Lymphoma 2009;50:604-11. [PubMed]
- American Cancer Society. Cancer Facts & Figures 2011-2013. Atlanta: GA, 2011.
- Rustagi T, Zarookian EI, Qasba O, et al. Chronic hepatitis C as a risk factor for colorectal adenoma. Int J Colorectal Dis 2014;29:75-80. [PubMed]
- Ferri C, Caracciolo F, Zignego AL, et al. Hepatitis C virus infection in patients with non-Hodgkin's lymphoma. Br J Haematol 1994;88:392-4. [PubMed]
- Levrero M. Viral hepatitis and liver cancer: the case of hepatitis C. Oncogene 2006;25:3834-47. [PubMed]
- Cho KR, Vogelstein B. Genetic alterations in the adenoma--carcinoma sequence. Cancer 1992;70:1727-31. [PubMed]
- Guillevin L, Lhote F, Cohen P, et al. Polyarteritis nodosa related to hepatitis B virus. A prospective study with long-term observation of 41 patients. Medicine (Baltimore) 1995;74:238-53. [PubMed]
- Johnson RJ, Couser WG. Hepatitis B infection and renal disease: clinical, immunopathogenetic and therapeutic considerations. Kidney Int 1990;37:663-76. [PubMed]
- Lai KN, Lai FM, Chan KW, et al. The clinico-pathologic features of hepatitis B virus-associated glomerulonephritis. Q J Med 1987;63:323-33. [PubMed]
- Lin CY. Clinical features and natural course of HBV-related glomerulopathy in children. Kidney Int Suppl 1991;35:S46-53. [PubMed]
- Fahal AH, el Razig SA, Suliman SH, et al. Gastrointestinal tract cancer in association with hepatitis and HIV infection. East Afr Med J 1995;72:424-6. [PubMed]
- Boguszaková L, Hirsch I, Brichácek B, et al. Absence of cytomegalovirus, Epstein-Barr virus, and papillomavirus DNA from adenoma and adenocarcinoma of the colon. Acta Virol 1988;32:303-8. [PubMed]
- Irby K, Anderson WF, Henson DE, et al. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975-2002). Cancer Epidemiol Biomarkers Prev 2006;15:792-7. [PubMed]
- Forde KA, Tanapanpanit O, Reddy KR. Hepatitis B and C in African Americans: current status and continued challenges. Clin Gastroenterol Hepatol 2014;12:738-48. [PubMed]
- Kim HS, Baik SJ, Kim KH, et al. Prevalence and risk factors of colorectal adenoma in 14,932 koreans undergoing screening colonoscopy. Korean J Gastroenterol 2013;62:104-10. [PubMed]
- Agrawal S, Bhupinderjit A, Bhutani MS, et al. Colorectal cancer in African Americans. Am J Gastroenterol 2005;100:515-23; discussion 514. [PubMed]
- Rex DX, Johnson DA, Anderson JC, et al. Colorectal Cancer Screening. Am J Gastroenterol 2009;104:739-50. [PubMed]


