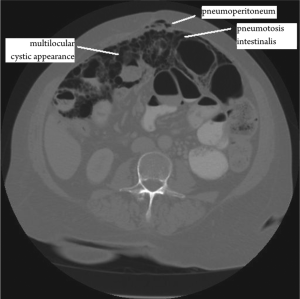
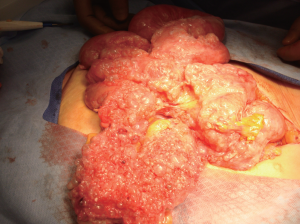
Approximately 150 cases of benign multicystic peritoneal
mesothelioma, with various presentations have been
reported since it was first described by Mennemeyer and
Smith in 1979 (
3-12). Upon extensive literature review, no
report of BMPM presenting with pneumoperitoneum and
pneumotosis intestinalis was identified. This disease is quite
rare (0.15/100,000 annually) which makes its diagnosis,
treatment, origin, and pathogenesis a unique clinical
challenge (
3).
Benign multicystic peritoneal mesothelioma lesions
usually occur in the peritoneum along the pelvic cul de
sac, uterus, and rectum, but may occasionally involve
the round ligament, small intestine, spleen, liver, kidney,
previous scars, or the appendix (
2,
1,
3,
4). Unlike malignant
mesothelioma, BMPM has not been shown to have an
association with asbestos exposure. In as many as half of
the cases, lesions have recurred within a few months to
years after resection (
1). Although it is considered benign,
rare cases have been reported to proceed to malignant
transformation (
5).
BMPM, also referred to as multilocular inclusion
cysts, occurs most frequently in young to middle-aged
premenopausal women (
1,
2). Rarely, it occurs in males
(
10,
14). The disease has been considered to be either a
hyperplastic reactive lesion or a benign neoplasm. Due to
its reported association with previous abdominal surgery
and endometriosis, some authors support the notion
of BMPM being a non-neoplastic reactive lesion (
2),
however, recurrence after partial resection and malignant
transformation resulting in death has been well documented
over the years (
5).
The lesions typically appear as single or multiple small,
thin-walled, translucent, unilocular cysts that may be
attached or free in the peritoneal cavity (
1). Extraperitoneal
locations such as the pleura , spermatic cord, and pericardium have been rarely reported (
2). Grossly the
cysts are most often seen attached and growing on the
surfaces of the pelvic cul de sac, uterus, and rectum in a
multilocular mass. The cystic fluid varies from yellow
to watery or gelatinous in consistency with the cytology
showing sheets of benign monomorphous mesothelial cells
(
2,
1). On microscopic examination BMPM cysts are lined
by a single layer of flattened to cuboidal mesothelial cells
which occasionally have a “hob-nail” appearance. In up to
one third of the cases, the lining of the cells can undergo
adenomatoid or squamous metaplasia (
1,
2).
Although pneumoper itoneum and pneumatosi s
intestinalis have a wide variety of differential diagnoses
ranging from benign to life threatening, these conditions
have never been reported as associated with benign
multicystic mesothelioma. The differential diagnosis of
BMPM includes a variety of malignant and benign lesions
that present as cystic or multicystic abdominal masses.
Cystic lymphangioma, cystic adenomatoid tumors, cystic
mesonephric duct remnants, endometriosis, mullerian
cysts involving the retroperitoneum, and cystic forms
of endosalpingiosis are several of the benign lesions that
should be considered in the differential (
11). Multilocular
cystic lymphangiomas are the most commonly confused
lesions with BMPM. Unlike BMPM, cystic lymphangiomas
usually occur in male children in extrapelvic regions. They
are usually found localized to the small bowel, omentum,
mesocolon, or retroperitoneum and contain chylous
contents. Unlike BMPM, they also have mural lymphoid
aggregates and smooth muscle unlike (
1,
11). Malignant
lesions to consider are malignant mesothelioma and serous
tumors that involve the peritoneum.
BMPM usually presents with vague lower abdominal
pain, mass, or both, but is also commonly diagnosed
incidentally upon laparotomy for other surgeries (
1). The
patient may also present with obstructive symptoms such as
nausea, bloating, or vomiting. Despite its relatively benign
process some patients may present with an acute abdomen
(
11). CT scans may be diagnostically beneficial but, as in
this case, can also indicate a more acute need for surgery as
actually necessary. Pre-operative fine needle core biopsies
have been reported to be of some benefit in the differential
diagnosis of BMPM (
11,
16). Cytologic features of peritoneal
washings in cases of BMPM have shown the washings
to be hypercellular with a population of mesothelial and
squamous metaplastic cells (
6). Ultimately, the diagnosis is
usually made by the pathologist after surgical resection has
been performed.
Due to its rarity, BMPM treatment options remain an
area of controversy and there is no streamlined treatment
plan. Currently aggressive surgical resection is the mainstay of treatment with palliative debulking and reoperation for
recurrence (
15,
11,
5). With up to 50 percent recurrence rates
and its malignant potential, debulking surgery does not
appear to be the most acceptable treatment option for these
patients. Patients may suffer from poorly controlled chronic
abdominal and pelvic pain (
15). Uncertain results have been
reported with patients receiving adjuvant chemotherapy
and/or radiation therapy (
5). Other approaches such
as sclerosive therapy with tetracycline, continuous
hyperthermic peritoneal perfusion with cisplatin, and
antiestrogenic drugs have been suggested (
11). The optimal
treatment may be cytoreductive surgery with peritonectomy
combined with perioperative intraperitoneal chemotherapy
to eliminate all gross and microscopic disease (
5). The goal
of this treatment regimen is to reduce the likelihood of
progression or recurrence.
Although the prognosis for BMPM is ver y good,
aggressive approaches to this disease should be considered.
Patients have a high likelihood of recurrence and repeat
surgeries are common. The intention of this report is to
increase the awareness of this disease entity and to consider
it whenever the patient’s presentation does not match that
of the working diagnosis. This patient presented without
peritoneal signs despite a CT scan that suggested a more
severe pathology. Before jumping into an exploratory
laparotomy based on imaging findings, surgeons should
trust our physical exam and pursue a more definitive
diagnosis. With a definitive diagnosis we can approach
the surgical issue in the most appropriate manner. In this
case, a diagnosis could have been made by a minimally
invasive technique such as a needle biopsy or a diagnostic
laparoscopy. Once a definitive diagnosis of BMPM is made,
then a single surgery should be the goal to eliminate all
gross and microscopic disease.