Liver-directed conversion therapy in metastatic colon cancer
Dr. Kemeny: A 37-year-old female had a fever for 1 week post-partum. Work-up identified an elevated alkaline phosphatase of 306 units/L (normal range, 30-88 units/L), associated with an estimated 24 liver lesions on ultrasound. A computed tomography (CT) scan showed a mass in the ascending colon, multiple regional nodes, and multiple liver metastases up to 6.5 cm × 6.4 cm in size, occupying at least 40% of the liver volume. Colonoscopy with biopsy showed a moderately differentiated adenocarcinoma with a KRAS G12D mutation. She had no significant family history. Genomic testing showed no microsatellite instability. She started treatment with FOLFOX6, and achieved a partial response over 4 months.
Dr. Abou-Alfa: Would anybody like to comment on the approach to therapy?
Dr. Olayan: I would have considered adding bevacizumab. The patient has bilobar disease; we are not sure if it is resectable, so we are not necessarily talking about conversion therapy at this point. While there are data for cetuximab as part of a conversion therapy regimen (1-3), there is a small trial showing conversion rate of over 50% using bevacizumab (4).
Dr. Saltz: I agree on Dr. Kemeny’s choice of chemotherapy, and I respectfully disagree about the use of bevacizumab. In the N01966 study, we had a randomized double blind trial of 1,400 patients who were first-line treated with oxaliplatin-based therapy (1). It was a two by two randomization: first patients were randomized to capecitabine plus oxaliplatin versus FOLFOX, and then they were randomized to bevacizumab or placebo. The response rate by the independent radiologist review was 38% in both the bevacizumab and placebo arms. By investigator adjudication they were also the same. Thus, there was no hint of improved response rate in the largest dataset we have on this topic.
Dr. Shamseddine: One may have to be careful about making conclusions from small studies that are uncontrolled, versus large controlled studies.
Dr. Saltz: In the N01966 study, in terms of progression-free survival, there was a modest improvement of 1.4 months that was statistically significant. This might have been undermined by early stoppage of bevacizumab in some patients. However, that would not have affected response rate, since we know from the OPTIMOX study that we get nearly all our response to FOLFOX in the first 3 months of treatment (5).
Dr. Abou-Alfa: If the KRAS status were wild-type, Dr. Kemeny would you have added cetuximab?
Dr. Kemeny: No, at this point I would not have added cetuximab. There are studies suggesting that cetuximab in this setting would increase the resection rate (2). But since I have a different approach in mind as you will see, I recommended initial FOLFOX, and planned to put the hepatic artery infusion (HAI) pump in shortly thereafter.
Dr. Saltz: While discussing anti-epithelial growth factor receptor (anti-EGFR) therapy, two therapies under consideration include panitumumab and cetuximab. There is evidence for modest benefit from the addition of panitumumab to oxaliplatin- or irinotecan-based chemotherapy in the metastatic setting (6,7). However, the question of whether panitumumab will yield benefit in the adjuvant or neoadjuvant setting remains unanswered. Multiple trials of FOLFOX6 with panitumumab have been completed or are ongoing (NCT01508000, NCT00647350), including one in the neoadjuvant setting (NCT0018107). In contrast, the MRC COIN or NORDIC VII studies, both large randomized multi-center cooperative group studies, were negative for the addition of cetuximab to 5-fluorouracil and oxaliplatin (8,9). In addition, when thinking about eradicating micrometastases and resecting the large lesions, both cetuximab and bevacizumab have not shown added benefit in large randomized phase III studies (10,11). These antibodies bring something to the table in unresectable metastatic disease, but not as much as we wanted to believe, and they certainly have major toxicity issues, especially the bevacizumab if you’re going into major surgery (12).
Dr. Abou-Alfa: Dr. Merhi, can you comment on the scans please?
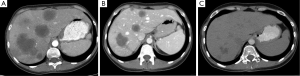
Dr. Merhi: There is extensive bilobar disease, an estimated forty percent of the total liver volume. You can also see the cauliflower appearance which is typical of colon cancer metastases.
Dr. Sidani: While the lateral aspect of the left lobe is relatively spared, this is definitely extensive disease, and I would not consider it surgically resectable at this point.
Dr. Merhi: In addition, many of the vessels are involved, which of course also makes it difficult to resect.
Dr. Abou-Alfa: Was the presumption that there was no extra-hepatic disease?
Dr. Kemeny: Yes. For patients with only liver disease, I treat all with the intent to go to surgery, no matter how extensive their liver disease.
Dr. Saltz: One has got to recognize that there is a different biology that is not understood between tumors that metastasize solely to the liver and those that metastasize elsewhere. In the setting of metastases to the liver alone, one needs to keep the surgical option open, in marked contradistinction to people with multi-organ disease, in whom surgery might not be possible.
Dr. Snyder: Has there been any research that helps distinguish those two conditions?
Dr. Kemeny: We have looked at KRAS mutation in our patients, and the expression was not different in patients who have liver only disease, although mutation correlated with more rapid progression and more widespread disease, particularly to the lung. Our study described evidence of metastases at 2 years as 27% in the KRAS wild type group versus 47.5% in KRAS mutant (13).
Dr. Kelsen: Dr. Massague and others have worked on such questions (14). Using an in vivo mouse model with the mouse essentially as a cell sorter, they passage a given cell line through mice via serial xenografts, then isolate tumor cells from a certain organ, to select cells with tropism for that organ. In one well-known example, they passaged MDA-MB-231 breast cancer cells to select clones with variable propensity for lung metastasis, then determined a transcription signature associated with lung metastasis (15). If you followed this reasoning out to its conclusion—that certain subpopulations home exclusively to the liver—you might say that colon cancer patients with liver-only metastases should be treated like hepatocellular carcinoma (HCC) and go to transplant, a strategy which has not been successful in this setting.
Dr. Mukherji: There is a study in Norway (www.clinicaltrials.gov NCT00294827) of transplant for colorectal cancer metastatic to the liver.
Dr. Kemeny: Within one month of starting systemic treatment with FOLFOX6, the patient derived clinical benefit, with decreased hepatic metastases and improved anemia. At her 8 week scan, her liver metastases were decreasing; for example from 4×6.4 to 2.9×4.7 cm2; her colonic mass had decreased from 5.9 to 2.7 cm. Later, by 20 weeks, the response started to slow down, with stabilization of colonic mass and liver lesions, which is typical of systemic chemotherapy response: you get a response over the first 4 months, then there is less of a response, or even progression.
Dr. Sidani: This patient’s disease remains unresectable. Any thoughts about intra-hepatic arterial infusion (HAI) pump therapy?
Dr. Kemeny: I agree. The patient did not have resectable disease. We discussed the case with our surgeons, who felt the disease was not resectable, and a HAI pump should be placed. The patient was treated on a phase II protocol of conversion therapy using floxuridine (FUDR) and dexamethasone administered by HAI combined with intravenous systemic therapy with irinotecan or oxaliplatin and bevacizumab. Because she had developed an allergy to oxaliplatin, this patient received FOLFIRI (fluorouracil, irinotecan, leucovorin) and bevacizumab.
Dr. Abou-Alfa: Dr. O’Reilly, what are your thoughts?
Dr. O’Reilly: The pump data in fit young patients with liver-only disease is compelling (16). There are no randomized data, but the single-institution data are so compelling that in this setting I think it’s a rational option.
Dr. Kemeny: We started HAI pump therapy 2 weeks after surgery. Within 3 months, one can see excellent reduction in tumor volume (Figure 1).
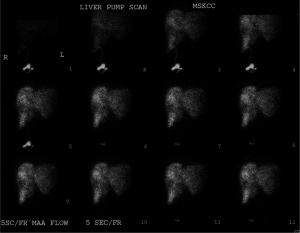
Dr. Abou-Alfa: What exactly do you do with the HAI pump therapy?
Dr. Kemeny: We continue to use systemic therapy. Systemic therapy was switched to FOLFIRI plus the HAI pump therapy. The pump is placed surgically subcutaneously in the abdomen (17). A catheter is fed through the gastroduodenal artery to perfuse the hepatic arteries. Flow scans are performed to ensure the pump flow is all contained within the liver and that there are no leaks. Figure 2 shows the perfusion scan of this patient. This scan uses technetium sulfur colloid and technetium microaggregated albumin to visualize the area of pump perfusion, ensuring that the entire liver is perfused without flow to adjacent structures. FUDR is used intrahepatically because this drug has excellent first pass extraction compared to other drugs. We continue to use systemic therapy. Systemic therapy was switched to FOLFIRI plus the HAI pump therapy. Dr. Sidani, how does this look to you now?
Dr. Sidani: The left lobe is now relatively clear, and if volumetric studies show that this is adequate for survival, then a resection can be done.
Dr. El-Naghy: You need 10 g/kg of remaining liver for adequate function. I doubt there is sufficient liver reserve remaining for this patient.
Dr. Kemeny: Unfortunately that was the case. The decision was made that surgical removal of tumor could only be done with a 2-stage surgery.
Dr. Abou-Alfa: Would any of the surgeons like to comment on what the 2-stage surgery involves?
Dr. Jamali: I agree. A 2-stage surgery is what this patient needs. I would target the left lobe first, either with a segmentectomy or a radiofrequency ablation, to clear the left lobe, and tie the right portal vein during that surgery, which would accomplish embolization on the right, and allow growth of the left, which is now clear of disease. Then, in the second stage, I would do an extended right hepatectomy to clear the remaining disease, in 8 to 10 weeks, once you have allowed adequate hypertrophy of the contralateral lobe.
Dr. Kemeny: Our surgeons did what you said, although they preferred doing the portal vein embolization first. They did RFA of segment 4A, segment II adjacent to left hepatic vein, RFA to segment IV deep to falciform ligament, wedge resection of the tip of segment II, which exhibited greater than 95% treatment effect; wedge resection of segment II, with greater than 80% treatment effect, and an RFA of two lesions of the anterior surface of the left lateral segment.
The second resection was done 3 months later, with a microwave ablation of the caudate tumor and caudate process tumor, again with a good response in the liver: right hepatic lobectomy, which showed 90% treatment effect, and wedge resection of segment 4, with 90% treatment effect. The primary was taken out when we put the HAI pump in. It was a poorly differentiated adenocarcinoma, with 2 out of 28 lymph nodes positive for disease. Five years later, the patient remains free of colon cancer.
Dr. Abou-Alfa: Dr. Kelsen, can you kindly comment on breast cancer and colorectal cancer in a 37-year-old woman?
Dr. Kelsen: The first thought that they had before was to check BRCA-1 and -2, which is not unreasonable. This combination of tumor types doesn’t leap to mind as BRCA-related. If she had had a signet cell colon cancer and a lobular breast cancer, then we would have checked CDH1 (18). The general theme is, in a young woman with two simultaneous cancers, there is likely a genetic problem—a driver mutation.
Dr. O’Reilly: You might also think of hereditary non-polyposis colon cancer (HNPCC), as there is some question whether there is an increased risk of breast cancer in these patients (19).
Dr. Abou-Alfa: Dr. Kemeny, what can you tell us about complications of the pump?
Dr. Kemeny: Our complication rate is low. Hepatic artery thrombosis may occur, in which case the pump doesn’t work. Extrahepatic perfusion is another problem, as flow to the liver is critical (Figure 2). If it happens that areas other than the liver are perfused, one may perform embolizations of the vessels feeding those sites, for example the stomach or pancreas. So you need good interventional radiologists to work with you in case you encounter those problems.
Dr. Mukherji: For how long did the patient receive therapy?
Dr. Kemeny: In general, we prefer to treat about 4 to 6 months treatment after resection. In this case, the patient is still cancer-free 5 years from resection and 6 years from starting therapy. It is important to stress that the pump is providing a protective effect to the liver, the most common site of recurrence.
Dr. Kelsen: There are two possibilities to explain liver recurrence: there may be seeding from other sites, as Dr. Newton et al. have suggested (20), or she may have had many sites of micrometastasis in the liver from the beginning, and over time they were appearing. Depending on which, the strategy would be different. The Norwegian group is trying to find those patients with disease restricted to the liver and transplant them (www.clinicaltrials.gov NCT00294827).
Dr. Kemeny: The EORTC study examined preoperative and post-operative chemotherapy in resectable patients (21). In their study, 90% of the patients had three or fewer liver metastases (patients with a good outcome). Among the eligible patients, there was an increase in progression free survival (PFS) with six cycles of FOLFOX before surgery and six cycles after. The EORTC study however showed no improvement in survival, although it was not powered to detect such a difference.
Their study was not a conversion study since all patients were resectable. There are several other studies that looked at conversion therapy. A randomized study comparing 6 months of induction therapy with FOLFOXIRI to FOLFIRI showed that patients who received FOLFOXIRI had an increase in PFS (9.8 vs. 6.8 months, P<0.01), and median overall survival (OS) (23.4 vs. 16.7 months, P=0.026). An increased resection rate was thought to be largely responsible for the survival advantage seen in the FOLFOXIRI group (22).
I would like to add that one has to limit the duration of preoperative therapy, as the oxaliplatin and the irinotecan can cause liver toxicity. You don’t necessarily want to treat to best response, but rather to resectability.
As for the role of the biologics, it continues to evolve. Regarding cetuximab we have to wait for the data from CALGB 80405 (www.clinicaltrials.gov NCT00265850).
Dr. Saltz: This is a good opportunity to explain the nuances of neoadjuvant and conversion therapies. Neoadjuvant treatment is treatment you choose to give to a patient when you already know you could operate, but you prefer to give the chemotherapy first. Conversion is different from the EORTC study: the decision has already been made that the patient’s disease is not resectable, and we are going to see what we can do to change that. I don’t think we should get hung up on whether we give the FOLFOX pre- or post-operatively on the basis of the EORTC. They did what I consider to be the worst choice, which is to split it with a big pause in the middle. I think it is best to either decide to give the therapy before or after surgery. The problem with giving it all prior, as Dr. Kemeny said, is that you can “beat up” the liver and cause problems with the safety of resection. It is for that reason that we prefer to do the operation, let the patient recover, then give 6 months of adjuvant therapy, similar to what we would do with a high-risk stage III patient. Nancy, your thoughts?
Dr. Kemeny: First let’s talk about why we consider liver-directed therapy. Fifteen percent of patients have liver metastases at diagnosis, and 60% develop liver metastases at follow-up. Liver metastases are perfused by the hepatic artery, while the rest of the liver is perfused by the portal vein. Some drugs are extracted by the liver during the first pass, and therefore may have less toxicity. The liver may be the only site of disease, with progression in a step-wise pattern, arriving via the portal vein. Therefore the idea is: if you can control the liver, you can control the progression of the rest of disease.
The drugs we can use for this from work done years ago by Ensminger (23), where he estimated the hepatic extraction and utility of several chemotherapeutic drugs. He showed the best was FUDR. Other drugs like mitomycin could be useful. For example, 5-fluorourcail (5-FU) has only a 5- to 10-fold increase in hepatic exposure, whereas FUDR has a 100- to 400-fold increase.
There was a large randomized study by the CALGB [9481] trying to answer the question, without crossover (since prior studies had had crossover) (24). At the time that study was started, 5-FU leucovorin was standard of care for systemic therapy. This study did show a survival advantage for the HAI arm, of 24 vs. 20 months for systemic chemotherapy. Importantly, the HAI arm and did not include any systemic therapy.
Dr. Abou-Alfa: However OS for metastatic disease treated with 5-FU leucovorin is not typically 20 months.
Dr. Kemeny: In this study, patient who failed 5-FU were offered some of the newer therapies like irinotecan or oxaliplatin. So the 20 months survival reflects what you get from cumulative use of these drugs.
Dr. Saltz: At the time of this study, 5FU was the standard front-line option, whereas irinotecan was being used only in 2nd-line outside of clinical trials. In hindsight, from the CAIRO study, that may be an acceptable option (25). As long as patients have access to and are exposed to all of the drugs available, whether we lump them together or give them sequentially probably doesn’t make a lot of difference. In this study, the favorable outcomes are probably due both do getting additional therapies in the 2nd-line setting, and the population of patients that present with liver-only disease probably has a better prognosis than patients who present with multi-focal disease.
Dr. Kemeny: I reiterate FUDR should not be given alone, because of the possibility of extra-hepatic disease. One can use almost full doses of systemic treatment along with the FUDR because the FUDR is being extracted almost entirely by the liver, so you don’t get systemic toxicity from FUDR, nor do you get systemic benefit from HAI FUDR alone.
Dr. Abou-Alfa: What were some of the reasons that patients did not make it to resection?
Dr. Kemeny: They may have had tumor located close to hepatic or portal vessels, or other technical reasons which would not allow enough normal liver to remain. Regarding adjuvant HAI after resection of liver metastases, we know 70% of patients will have recurrence, of which 50% will recur in the liver. We did a randomized study looking at HAI + systemic versus systemic chemotherapy alone. We noted a 2-year survival increase of 85% vs. 69% with HAI + systemic versus systemic chemotherapy alone. After a median follow-up of 10 years, 10-year survival was 41% vs. 27% HAI + systemic and systemic chemotherapy alone, respectively (26,27). The important thing is we were decreasing liver recurrence. In this study, only 26% of patients recurred in the liver with HAI + systemic versus 56% with systemic chemotherapy alone.
Dr. Kelsen: The difference in the curves for OS is much smaller than for hepatic survival. Therefore there is no question you’re having an impact on all the patients, but particularly on the hepatic-only patients.
Dr. Saltz: It’s a molecular holy grail of regional therapy: if you could determine the identity of the patients who will recur locally versus systemically, that would change the enthusiasm for regional therapy.
Dr. O’Reilly: There are durable, complete remissions with regional therapy, which you don’t see with systemic-only therapy.
Dr. Kelsen: Surgeons talk about the learning curve of a new procedure. What’s the number of cases for the medical oncologist supervising pump therapy?
Dr. Kemeny: Surgeons say about five cases. We have converted how to give HAI therapy into a formula. If you follow the formula, most medical oncologists could do this treatment.
Dr. O’Reilly: It’s not easy, but it’s doable, with Dr. Kemeny’s guidance.
Dr. Abou-Alfa: Can anyone from the AUB team comment on what would be the major challenges to start a pump program?
Dr. Mukherji: One of the major challenges is the price of the pump and reimbursement as this is not considered a standard of care. We would need to have that learning curve and quite a lot of help and assistance, but this should not be a concern.
Dr Snyder: To summarize, bevacizumab added to oxaliplatin and 5-FU leads to an increase in PFS but not OS in metastatic colon cancer. Cetuximab may increase response rate and OS when added to FOLFIRI (27). Cetuximab and panitumumab are ineffective in extended RAS-mutant colon cancers. With respect to liver metastases in colon cancer, 15% of patients have liver metastases at diagnosis, and 60% develop liver metastases during the course of their disease. Conversion therapy aims to “convert” unresectable lesions to resectability, whereas with neoadjuvant therapy, systemic therapy is given before the removal of lesions that are resectable at presentation. In most randomized trials of liver-directed therapy with HAI after liver resection, hepatic progression-free survival and progression-free survival are improved for HAI therapy when compared to systemic alone. Extraction of FUDR by the liver makes this medication particularly appropriate for this approach, and permits the simultaneous administration of systemic chemotherapy to treat other sites of micrometastasis.
Acknowledgements
This case was presented at the MSKCC/American University of Beirut/ National Guard Hospital, Riyadh case conference in March 2013. This conference is supported by endowment gift of Mrs. Mamdouha El-Sayed Bobst and the Bobst Foundation.
Disclosure: The authors declare no conflict of interest.
References
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9. [PubMed]
- Adam R, Aloia T, Lévi F, et al. Hepatic resection after rescue cetuximab treatment for colorectal liver metastases previously refractory to conventional systemic therapy. J Clin Oncol 2007;25:4593-602. [PubMed]
- Kemeny NE. Treatment of metastatic colon cancer: "the times they are A-changing J Clin Oncol 2013;31:1913-6. [PubMed]
- Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol 2010;11:38-47. [PubMed]
- Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol 2006;24:394-400. [PubMed]
- Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023-34. [PubMed]
- Peeters M, Price TJ, Cervantes A, et al. Final results from a randomized phase 3 study of FOLFIRI {+/-} panitumumab for second-line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:107-16. [PubMed]
- Tveit KM, Guren T, Glimelius B, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol 2012;30:1755-62. [PubMed]
- Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2103-14. [PubMed]
- Allegra CJ, Yothers G, O'Connell MJ, et al. Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol 2013;31:359-64. [PubMed]
- Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 2012;307:1383-93. [PubMed]
- Scappaticci FA, Fehrenbacher L, Cartwright T, et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol 2005;91:173-80. [PubMed]
- Kemeny NE, Chou JF, Capanu M, et al. Association of KRAS mutation with worse recurrence-free survival and site of metastatic progression after resection of hepatic colorectal metastases. J Clin Oncol 2013;31:abstr 3609.
- Nguyen DX, Massagué J. Genetic determinants of cancer metastasis. Nat Rev Genet 2007;8:341-52. [PubMed]
- Minn AJ, Gupta GP, Siegel PM, et al. Genes that mediate breast cancer metastasis to lung. Nature 2005;436:518-24. [PubMed]
- Kemeny NE, Melendez FD, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol 2009;27:3465-71. [PubMed]
- Kemeny N, Fata F. Hepatic-arterial chemotherapy. Lancet Oncol 2001;2:418-28. [PubMed]
- Daly MB, Axilbund JE, Buys S, et al. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw 2010;8:562-94. [PubMed]
- Buerki N, Gautier L, Kovac M, et al. Evidence for breast cancer as an integral part of Lynch syndrome. Genes Chromosomes Cancer 2012;51:83-91. [PubMed]
- Newton PK, Mason J, Bethel K, et al. Spreaders and sponges define metastasis in lung cancer: a Markov chain Monte Carlo mathematical model. Cancer Res 2013;73:2760-9. [PubMed]
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-16. [PubMed]
- Masi G, Vasile E, Loupakis F, et al. Randomized trial of two induction chemotherapy regimens in metastatic colorectal cancer: an updated analysis. J Natl Cancer Inst 2011;103:21-30. [PubMed]
- Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Semin Oncol 1983;10:176-82. [PubMed]
- Kemeny NE, Niedzwiecki D, Hollis DR, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol 2006;24:1395-403. [PubMed]
- Koopman M, Antonini NF, Douma J, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet 2007;370:135-42. [PubMed]
- Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med 2005;352:734-5. [PubMed]
- Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:1065-75. [PubMed]