The functional and prognostic implications of regulatory T cells in colorectal carcinoma
Treg introduction
In order for the immune system to function properly (e.g., robust immune defense, immune surveillance and immune homeostasis), a complex controlling mechanism must be on board. Both T and B cells need to undergo rigorous selection during maturation before being released to the periphery. However, intrinsic mechanisms of T-cell elimination, including activation-induced cell death and anergy, may not alone prevent autoimmune disease. Among the immune cell populations and subpopulations, regulatory T cells (Tregs) have been accepted as a developmentally and functionally distinct group that plays a major role in maintenance of self-tolerance, largely due to their ability to suppress responses mediated by other populations of T cells. Since their discovery in the 1960s as suppressive T cells, Tregs have been extensively studied in a wide range of both physiological and pathological conditions in mouse and man (1). In fact, Tregs represent a peripheral system to maintain self-tolerance and prevent over-exuberant immune responses (2). However, Tregs may also hamper effective anti-tumor immune response in cancer patients, considering that most tumor-asociated antigens (TAAs) identified to date are antigenically normal self-constituents (3).
Recent studies have suggested that human Tregs are functionally and phenotypically diverse (4). In fact, Tregs can be classified into a number of subtypes (5,6), including the well-known CD4+ CD25+ natural Tregs (nTreg) (7), which are self-antigen reactive, develop in the thymus in early neonatal development and emerge into peripheral tissues where they suppress the activation of self-reactive T cells in a T/T and T/APC contact dependent and cytokine independent manner. By contrast, induced Tregs (iTreg) are generated in the periphery and function mainly to maintain homeostatic control, which suppress through both T/T and T/APC contact and through production of interleukin 10 (IL-10) and TGF-beta (8). nTregs comprise 5-10% of the circulating CD4+ population, whilst circulating and tissue iTreg numbers depend on anatomic location as well as specific inflammatory environmental conditions (2). Both nTreg and iTreg express the transcription factor forkhead box P3 (Foxp3) (9). Meanwhile, there are Foxp3 negative suppressor T cells, including Tr1, Th3 cells, CD8+CD28+/-, and Qa1-restricted T cells. However, the contribution of these T cells to self-tolerance, immune homeostasis as well as preventing autoimmunity is not well defined (10). Our current discussion is mainly focused on Foxp3 positive Tregs.
In the past decade, much effort has been devoted in finding molecular markers that uniquely define Tregs (11). The initial characteristic phenotype of Tregs is CD4+ CD25+. However, CD25 expression cannot be used in human studies as peripheral blood isolated from an outbred human population contains up to 30% CD4+ CD25+ T cells, only 1-2% of cells with the highest CD25 expression have been shown to be functionally suppressive and can be considered Tregs (4). One of the cornerstones in the Treg research was the identification of Foxp3 as the unique phenotypic and functional marker (9). In fact, Foxp3 is specifically required for Treg cell development and is sufficient to activate a program of suppressor function in peripheral nonregulatory CD4+ T cells, therefore Foxp3 is a “master regulator” gene for this critically important subset of T cells (12). Being an X chromosome-encoded transcription factor, Foxp3 is indispensible for both development and function of Tregs. Mice mutated in Foxp3 as well as patients with immune dysregulation polyendocrinopathy, enteropathy, and X chromosome-linked syndrome (IPEX) result in the development of complex autoimmune diseases due to the deficiency of Tregs. Therefore, constitutive expression of Foxp3 is fundamental for the maintenance of the suppressive function of Tregs (10). Recently, it was found that Tregs are not the only cells that express FoxP3, some non-regulatory T cells, or even tumor cells also show the expression (4,13).
Besides Foxp3, there are other surface markers have been found expressed on Tregs, including CTLA-4 (14), LAG3 (15), GITR (16), HLA-DR (17), ICOS (18), and CD127 (19). For example, CTLA-4 is critical in activity of Tregs (10). However, these have not enabled homogenous Treg purification as most of them are also upregulated during T cell activation and are thus present on effect T cells (Teff) (20). In fact, the combination of the CD45RA and HLA-DR markers could divide Tregs into three distinct subpopulations: naïve Tregs (CD45RA+, HLA-DR-), memory Tregs (CD45RA-, HLA-DR-) and memory/activated Tregs (CD45RA-, HLA-DR+) (21). And also, following antigenic stimulation, CD45RA+ Foxp3lo naïve Tregs seem to differentiate into CD45RO+ Foxp3hi effector Tregs, which exert strong suppression and then die by apoptosis (4).
The main function of CD4+ CD25+ Tregs is the maintenance of tolerance to self-antigens, but should not interfere with the induction of pathogen-specific protective immune responses. That is to say, Tregs have a major importance in modulating host responses to tumor and infections, in preventing transplant rejection, and in inhibiting the development of autoimmunity and allergy (22). Indeed, Tregs can be defined as a T-cell population that functionally suppresses an immune response by influencing the activity of another cell type (23). For example, Tregs can suppress the activation, proliferation and effector functions (such as cytokine production) of a wide range of immune cells, including CD4+ and CD8+ T cells, natural killer (NK) and NKT cells, B cells and antigen-presenting cells (APCs) in vitro and in vivo (4). Therefore, Tregs play critical role in inflammation resolution and restoration of immune homeostasis. However, with this activity, in some cases Tregs also protected virus or carcinoma from immune clearance and promoted these diseases development (24).
Tregs are increased and enriched in colorectal cancer patients
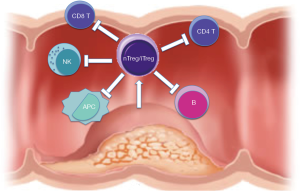
Since the description and characterization of Treg population, numerous studies have documented an increase of Tregs in the circulation, draining lymph nodes, and at the tumor sites of patients with malignancy, including head and neck, GI tract, lung, pancreas, breast and skin cancers, as well as lymphoma and leukemia (25). The tumor microenvironment might contain thymus-derived nTreg cells, expanded and converted nTregs, and locally differentiated and expanded iTregs (23) (Figure 1). Indeed, compelling studies in mice and human have demonstrated that many cancers can induce the proliferation of Tregs and/or promote their generation from naive T cells, resulting in the accumulation of these cells in the tumor beds and in the periphery. Importantly, the elimination and/or functional inactivation of tumor-induced Tregs can promote antitumor immunity and enhance the efficacy of immunotherapy (26). There are a couple of possible mechanisms that may explain the enriched Treg in tumor site. For example, Tregs are preferentially recruited to the tumor site. Tregs express receptors for chemokine such as CCR4, CCR5, CXCR4, and CCR10 that could induce their migration toward the tumor (27). And also, Tregs can be specifically expanded by cancer-derived factors or as a physiological defense phenomenon against inflammation induced by cancer, such as TGF-beta, IL-10 and H-ferritin (25). Furthermore, naive and memory conventional T cells can convert into Tregs with the help of immature antigen-presenting cells or myeloid-derived suppressor cells (MDSC). It has been shown in several animal models and in humans that tumors can convert naïve CD4+ cells into Tregs and expand tumor-specific Tregs (28). Lastly, naturally occurring Tregs are more resistant to oxidative stress than conventional T cells, possibly contributing to their survival in stressful tumor environments (27).
Colorectal cancer (CRC) is one of the most common cancers worldwide and a major cause of cancer-related death (29). Therefore, it is meaningful to explore the Tregs and their possible role in CRC, as well as the potential significance in the therapeutic strategies. It was shown that in colorectal tumors regional lymph nodes remain heavily infiltrated by Treg cells (1). Moreover, for patients with CRC, increased numbers of Tregs had been shown in peripheral blood, tumor-draining lymph node, and tumor site, and these Tregs could actively migrate to the site of immune activity (23,29,30). In fact, the microbial environment promotes colonic Treg differentiation and contributes to immune homeostasis in the colon (31). Treg generation in colon is necessary to maintain intestinal immune tolerance. Furthermore, the commensal mibrobiota are important for mucosa Treg abundance, because in the colon germ-free mice or vancomycin-treated mice the Tregs are severely reduced. In addition, the colonic DCs show probiotics capability, which can repress the expression of LPS response genes as well as inhibit macrophage activation due to hyporesponsiveness to TLR stimulation, therefore induce Treg differentiation. On the other hand, these Tregs are specific TAA reactive, since in patients with CRC, carcinoembryonic antigen, telomerase, HER2/neu,and MUC-1 reactive Tregs were detected (5). In fact, TAA-specific Tregs are predominantly present in the blood of CRC patients but are not dectectable in healthy individuals (32).
The function of Tregs in CRC is controversial
It is thought that the failure to mount an efficient immune response is due to a hostile tumor microenvironment dominated by immunosuppressive cells. Tregs have received special attention because of their vigorous inhibitory action on effector T cells, and high Treg numbers enable cancer cells to evade the host immune response (20,28). The most significant consequence of Tregs increased and enriched in tumor patient is compromising the antitumor immunity through production of cytokines, such as TGF-β and IFN-γ, or by direct cell-to-cell contact (33). And also it was shown that infiltration of tumors by Tregs confers growth and metastatic advantages by inhibiting antitumor immunity and by production of receptor activator of NK-κB (RANK) ligand, which may directly stimulate metastatic propagation of RNAK-expressing cancer cells (5). Interestingly, Tregs in tumor patients were found to be highly specific for a distinct set of tumor associated antigens (TAAs), suggesting that Tregs exert T cell suppression in an antigen-selective manner (32). Moreover, TAA-specific Tregs might suppress APC and T-cell function by producing IL-10, and suppress NK cell function by producing TGF-beta, therefore dampen adaptive and innate immunity against cancer. As most tumor antigens are self-antigens, Treg-medicated suppression of TAA-reactive cells has been proposed as a potential mechanism to explain the failure of antitumor immunity (23).
The role for Tregs in tumor immunity was first discovered when antitumor T-cell immune responses were enhanced in mice after this Treg subpopulation was inhibited in vivo by anti-CD25 monocloncal antibody (34). Since Treg cells are essential to tumor-induced peripheral tolerance and are a barrier to tumor immunotherapy (35), and a high number of intratumoral Foxp3 cells has been associated with a higher risk of recurrence and poor overall survival of patients with solid neoplasm (36), moreover, in murine tumor models depletion of Tregs before tumor inoculation promotes tumor rejection and inhibition of tumor growth (25), it is reasonable to assume that the higher levels of Tregs in CRC can similarly inhibit anti-tumor immune responses and may contribute to tumoral immune escape and disease progression. Indeed, one research showed that elevated peritumoral numbers of Foxp3 Treg cells were associated with advanced-stage tumors and poorer overall survival (27). It was shown that the presence of CRC drives the activity of Tregs and accompanying suppression of CD4 T cells responses to tumor-associated antigens (TAA), which is associated with tumor recurrence at 12 months after the resection. It was also shown that the excision of the primary CRC leads to a normalization of the Treg population (30). In addition, in colorectal cancer patients only effector/memory T cell responses against TAAs strongly increased after Treg depletion (32). Furthermore, it was demonstrated that CRC could secret CCL5 which recruits Tregs to tumors through CCL5/CCR5 signaling and enhances their ability to kill antitumor CD8 T cells via inducing apoptosis, thereby promotes an immunosuppressive tumor microenvironment that helps in CRC progression (29).
However, although it is clear that Tregs are increased in CRC patients, the significance of this phenomenon is rather controversial. For example, in gastric cancer it was shown that high Treg density in the sentinel lymph node (SLN) could suppress the antitumor immune response within SLNs and eventually promote metastatic dissemination of tumors, whereas in CRC the Treg expression in SLNs correlated with increased tumor protection and survival and was indicative of a successful immune response (37). In fact, many researches show a different significance of Tregs in CRC, therefore the prognostic impact of Treg in CRCs becomes a matter of debate. One study demonstrated that in CRC intratumoral Tregs suppressed matrix metalloproteases in the presence of IL-17, which were associated with suppressed matrix metalloprotease activities and decreased metastases (24). In another study, it was found that the density of Foxp3+ Treg cells infiltrating CRCs was significantly higher in parallel with enhanced number of CD8+ T cells in CRCs with high-level microsatellite instability (MSI-H), the CRC subtype that rarely develops metastases in distant organs and has a comparably good prognosis (38). deLeeuw et al. pointed out that tumor site had a major influence Foxp3+ T cells were associated with poor prognosis in hepatocellular cancer but generally good prognosis in CRC, whereas other cancer types were inconsistent or understudied. They further noted that in CRC, Foxp3+ T cells may inhibit tumor-promoting inflammatory responses to gut microbes, which could explain their association with favorable outcomes in these and similar contexts (11).
In fact, animal modeling suggests that, at least in early stages of CRC, Tregs may have a protective function by suppressing cancer-associated inflammation, a benefit that is lost later through conversion of the Tregs to a pro-inflammatory phenotype (39). Indeed, by dampening the inflammation in a mouse model for chronic microbial inflammation Tregs actually prevented CRC, so appear to influence immunoediting and malignant progression (40). Having a high amount of Tregs, repressing microbe induced inflammation, could mean a protection not only by hindering the development of cancer in the colorectal epithelium, but also by preventing tumor growth. Besides, bacteria and bacterial products could also potentially aid to sustain the activity of the tumor immune response (41). Therefore Tregs’ action in CRC prognosis depends on the different tumor stage. In the early carcinogenesis, CRC is associated with prolonged pro-inflammatory insults driven by gastrointestinal bacteria, and Tregs are instrumental in limiting the local inflammation that ultimately leads to cancer. The abundant presence of Foxp3+ Treg within inflammatory infiltrates into tumor tissues signifies control of carcinogenesis, ameliorates proinflammatory signals and is associated with improved outcome; when the tumor proceeds to block functions of accumulating anti-tumor effector cells and instigates a massive conversion of conventional T cells to iTreg with powerful and varied suppressive capabilities. iTreg are resistant to apoptosis, accumulate and promote tumor escape. Their numbers increase as tumor progresses, and therefore they could serve as surrogate markers of poor outcome (13).
The prognostic impact of Treg in CRCs is also a matter of debate. One report showed elevated peritumoral numbers of Foxp3 Treg cells are associated with advanced-stage tumors and poorer overall survival (27). However, other reported that high numbers of intratumoral Foxp3+ Tregs are associated with better survival of CRC patients (30). The author explained that the role of Tregs may depend on the type of immune response present in the tumor microenvironment. When inflammatory cells that promote tumor progression dominate the immune response, Tregs may be beneficial in suppressing this process; However, in the case where the immune response is dominated by T cells, Tregs may promote disease progression by suppressing their anti-tumor effects (30). Another research showed Foxp3 high intensity in the microenvironment of SLNs was correlated with lack of migration of the tumor to the downstream lymph nodes. Concomitant high frequency of Foxp3 and tumor regression indicated that, in the context of the CRC, Tregs are not playing an immunosuppressor role. Instead, their presence may indicate homeostatic control of a robust immune response (42).
Potential therapeutic strategies to CRC
CRC is the fourth commonly diagnosed cancer, with an estimated 500,000 deaths per annum. Despite advances in preoperative imaging, surgical technique and neoadjuvant chemotherapy, approximately 40% to 50% of patients have disease recurrence following potentially curative surgery, highlighting the demand for better treatment. Targeting Tregs may offer an important therapeutic strategy as an adjunct to treatment of patients (30). In fact, it has demonstrated that Treg-mediated immunosuppression is one of the crucial tumor immune-evasion mechanisms and the main obstacle of successful tumor immunotherapy (23). Treg cells are essential to tumor-induced peripheral tolerance and are a barrier to tumor immunotherapy. Some cytotoxic agents deplete Treg cells systemically, and Treg modulation in patients with CRCs might boost antitumor immunity or the response to immunotherapy (27,35). Terme et al. showed that specific blockade of the VEGF-A/VEGFR axis by an anti-VEGF mAb is sufficient to inhibit Treg accumulation in mouse spleen and tumor in the CRC model as well as in peripheral blood of patients with CRCs. In addition, Treg depletion from peripheral blood of patients with CRCs unmasked CD4 and CD8 T cell responses against tumor-associated antigens in vitro (27). In another report, depletion of Tregs in the peripheral blood of patients with CRC was shown to boost CD4+ T cell responses to TAAs (32).
However, the benefit or hazard of Tregs increase in CRC is currently under debate. Since some reports concluded that a high density of Foxp3+ Tregs in tumor tissue is associated with improved survival, it is predicted that targeting the pathogenic cross-talk between Treg and mast cells to allow recovery of Treg anti-inflammatory functions will help to control CRC (43). On the other hand, use of Tregs for immunotherapy has a solid preclinical database, and emerging data support the safety and efficacy of Treg immunotherapy protocols in patients whose clinical scenario requires induction of clinical tolerance, such as allograft tolerance, atopic disease, autoimmune disease, as well as acute inflammatory disease (2). Nevertheless, since there are differences between Tregs and Effector T cells in the repertoires of recognized TAAs, alternative option is using selected sets of TAAs for tumor vaccinations that induce optimal effector T-cell responses but minimal Treg activity, which may improve the efficacy of vaccination protocols without the need for depletion of Tregs (39).
Concluding remarks
CRC is an important cancer that has a significant impact in the health care outcomes. It has already confirmed that Tregs are increased and enriched in CRC patients. Yet its actions and the significance on prognosis of CRC patients are controversial, and it seem Tregs have different influence at the different stage of CRC development. Therefore, it is imperative to clarify their activity, so as to adopt different strategies to manipulate Tregs, eventually improve the patients survival.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer 2007;7:880-7. [PubMed]
- Singer BD, King LS, D'Alessio FR. Regulatory T cells as immunotherapy. Front Immunol 2014;5:46. [PubMed]
- Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol 2014;27:1-7. [PubMed]
- Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010;10:490-500. [PubMed]
- Byrne WL, Mills KH, Lederer JA, et al. Targeting regulatory T cells in cancer. Cancer Res 2011;71:6915-20. [PubMed]
- Yoshii M, Tanaka H, Ohira M, et al. Expression of Forkhead box P3 in tumour cells causes immunoregulatory function of signet ring cell carcinoma of the stomach. Br J Cancer 2012;106:1668-74. [PubMed]
- Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995;155:1151-64. [PubMed]
- Chaudhry A, Rudra D, Treuting P, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 2009;326:986-91. [PubMed]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057-61. [PubMed]
- Haque M, Fino K, Lei F, et al. Utilizing regulatory T cells against rheumatoid arthritis. Front Oncol 2014;4:209. [PubMed]
- deLeeuw RJ, Kost SE, Kakal JA, et al. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res 2012;18:3022-9. [PubMed]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003;4:330-6. [PubMed]
- Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why? Semin Cancer Biol 2012;22:327-34. [PubMed]
- Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008;322:271-5. [PubMed]
- Camisaschi C, Casati C, Rini F, et al. LAG-3 expression defines a subset of CD4(+)CD25(high)Foxp3(+) regulatory T cells that are expanded at tumor sites. J Immunol 2010;184:6545-51. [PubMed]
- Levings MK, Sangregorio R, Sartirana C, et al. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med 2002;196:1335-46. [PubMed]
- Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol 2006;176:4622-31. [PubMed]
- Ito T, Hanabuchi S, Wang YH, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity 2008;28:870-80. [PubMed]
- Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 2006;203:1693-700. [PubMed]
- Banham AH, Powrie FM, Suri-Payer E. FOXP3+ regulatory T cells: Current controversies and future perspectives. Eur J Immunol 2006;36:2832-6. [PubMed]
- Chevalier MF, Didier C, Petitjean G, et al. Phenotype Alterations in Regulatory T-Cell Subsets in Primary HIV Infection and Identification of Tr1-like Cells as the Main Interleukin 10-Producing CD4+ T Cells. J Infect Dis 2015;211:769-79. [PubMed]
- Teng MW, Ngiow SF, von Scheidt B, et al. Conditional regulatory T-cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Cancer Res 2010;70:7800-9. [PubMed]
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 2006;6:295-307. [PubMed]
- Wang Q, Feng M, Yu T, et al. Intratumoral regulatory T cells are associated with suppression of colorectal carcinoma metastasis after resection through overcoming IL-17 producing T cells. Cell Immunol 2014;287:100-5. [PubMed]
- Tokuno K, Hazama S, Yoshino S, et al. Increased prevalence of regulatory T-cells in the peripheral blood of patients with gastrointestinal cancer. Anticancer Res 2009;29:1527-32. [PubMed]
- Alizadeh D, Larmonier N. Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res 2014;74:2663-8. [PubMed]
- Terme M, Pernot S, Marcheteau E, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res 2013;73:539-49. [PubMed]
- Medina-Echeverz J, Fioravanti J, Zabala M, et al. Successful colon cancer eradication after chemoimmunotherapy is associated with profound phenotypic change of intratumoral myeloid cells. J Immunol 2011;186:807-15. [PubMed]
- Chang LY, Lin YC, Mahalingam J, et al. Tumor-derived chemokine CCL5 enhances TGF-β-mediated killing of CD8(+) T cells in colon cancer by T-regulatory cells. Cancer Res 2012;72:1092-102. [PubMed]
- Betts G, Jones E, Junaid S, et al. Suppression of tumour-specific CD4+ T cells by regulatory T cells is associated with progression of human colorectal cancer. Gut 2012;61:1163-71. [PubMed]
- Zeng H, Chi H. Metabolic control of regulatory T cell development and function. Trends Immunol 2015;36:3-12. [PubMed]
- Bonertz A, Weitz J, Pietsch DH, et al. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest 2009;119:3311-21. [PubMed]
- Kim KJ, Lee KS, Cho HJ, et al. Prognostic implications of tumor-infiltrating FoxP3+ regulatory T cells and CD8+ cytotoxic T cells in microsatellite-unstable gastric cancers. Hum Pathol 2014;45:285-93. [PubMed]
- Izhak L, Ambrosino E, Kato S, et al. Delicate balance among three types of T cells in concurrent regulation of tumor immunity. Cancer Res 2013;73:1514-23. [PubMed]
- Balachandran VP, Cavnar MJ, Zeng S, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med 2011;17:1094-100. [PubMed]
- Villanacci V, Not T, Nascimbeni R, et al. Gastrointestinal Foxp3 expression in normal, inflammatory and neoplastic conditions. Pathology 2011;43:465-71. [PubMed]
- Lee HE, Park DJ, Kim WH, et al. High FOXP3+ regulatory T-cell density in the sentinel lymph node is associated with downstream non-sentinel lymph-node metastasis in gastric cancer. Br J Cancer 2011;105:413-9. [PubMed]
- Michel S, Benner A, Tariverdian M, et al. High density of FOXP3-positive T cells infiltrating colorectal cancers with microsatellite instability. Br J Cancer 2008;99:1867-73. [PubMed]
- Khazaie K, Bonertz A, Beckhove P. Current developments with peptide-based human tumor vaccines. Curr Opin Oncol 2009;21:524-30. [PubMed]
- Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol 2008;20:241-6. [PubMed]
- Ling A, Edin S, Wikberg ML, et al. The intratumoural subsite and relation of CD8(+) and FOXP3(+) T lymphocytes in colorectal cancer provide important prognostic clues. Br J Cancer 2014;110:2551-9. [PubMed]
- Matera L, Sandrucci S, Mussa A, et al. Low Foxp3 expression in negative sentinel lymph nodes is associated with node metastases in colorectal cancer. Gut 2010;59:419-20. [PubMed]
- Blatner NR, Bonertz A, Beckhove P, et al. In colorectal cancer mast cells contribute to systemic regulatory T-cell dysfunction. Proc Natl Acad Sci U S A 2010;107:6430-5. [PubMed]


