Capecitabine-induced leukocytoclastic vasculitis under neoadjuvant chemotherapy for locally advanced colorectal cancer
Background
This is the first report of capecitabine-induced leukocytoclastic vasculitis and the first report that illustrates the feasibility of controlling cutaneous vasculitis with an oral steroid without interrupting the curative treatment protocol that includes the capecitabine which was the culprit drug.
Case presentation
A 61-year-old Caucasian female with a past medical history of hypertension, rheumatoid arthritis, osteoarthritis, and hyperlipidemia presented with a new onset rectal bleeding. A colonoscopy showed a circumferential, centrally ulcerating mass starting at 2 cm from the anal verge, approaching the sphincter and extending to 8 cm from the anal verge. The mass was palpable from the vagina on pelvic exam. An MRI of the pelvis confirmed a 4.5 cm mass with extension through the rectal wall that was inseparable from the vaginal cuff. Additionally, sub-centimeter perirectal nodes were seen. A CT of the chest showed no evidence of metastatic disease. Biopsy demonstrated a moderately differentiated adenocarcinoma. Final clinical staging was tumor 4 node 2 metastases 0 (T4N1M0) in keeping with locally advanced rectal cancer.
The patient began the standard concurrent chemoradiation for locally advanced rectal cancer (1), using capecitabine and 50.4 Gy of radiation to the pelvis over a 5.5-week period, with planned surgical resection of the rectal tumor 6-8 weeks after the completion of the chemoradiation therapy. Capecitabine was given in a dose of 825 mg/m2 twice daily. Five days after initiating the concurrent chemoradiation therapy, the patient developed a pruritic maculopapular rash over her lower and upper limbs.
Investigations
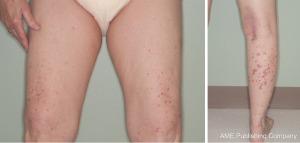
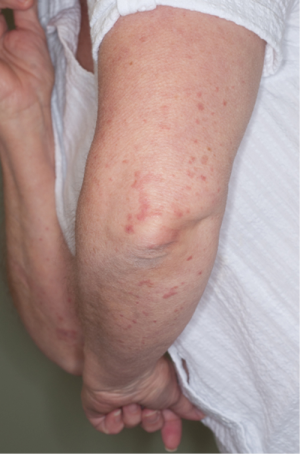
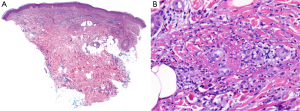
An initial physical examination revealed non painful, palpable, maculopapular lesions on the bilateral upper and lower extremities, more prominently in the lower limbs (Figures 1,2). The patient denied any systemic symptoms, specifically fever; a review of the system was positive for dysuria. Initial blood work, including a complete blood count and liver function test, was within normal limits. Based on the physical examination, vasculitis was suspected and a punch skin biopsy was obtained. Histopathologic examination revealed skin with a vasculocentric inflammatory infiltrate comprised of neutrophils, lymphocytes and histiocytes with associated dermal hemorrhage (purpura) and fibrinoid necrosis of the vessel wall and leukocytoclasis, confirming the diagnosis of leukocytoclastic vasculitis (Figure 3A,B). Given the patients dysuria, a urinalysis (UA) was obtained to rule out systemic/renal involvement. The UA was positive for leukocyte esterase, white cells 12 WBC per high power field (HPF) (normal, 0-2), and 4 RBC per HPF (normal, 0-2). There was no evidence of proteinuria or RBC casts. Urine culture was positive for gram negative rods. Other markers of systemic vasculitis, including ANA, ANCA, rheumatoid factor, and ESR, were all within normal limits.
Differential diagnosis
The possible etiologies for leukocytoclastic vasculitis, in general, are numerous. The critical distinction is between primary autoimmune (idiopathic) and secondary etiology. Potential entities to consider in the current case were autoimmune (given the patient’s history of rheumatoid arthritis), infection/bacteremia related to the dysuria and possible urinary tract infection, thrombocytopenia due to the possibility of thrombocytopenia induced by chemotherapy, paraneoplastic vasculitis (secondary to her underlying malignancy), or drug-induced vasculitis. The latter was favored due to the temporal relationship between the initiation of the capecitabine and the onset of the rash.
Treatment
A urinary tract infection was initially treated with ciprofloxacin, 500 mg twice daily for 10 days. Three days after the ciprofloxacin was started the patient reported ongoing abdominal cramps with the ciprofloxacin and was switched to nitrofurantoin 100 mg twice daily twice daily for 10 days. Capecitabine was withheld immediately due to the clinical suspicion of being the culprit drug, and daily radiation treatment was continued with no interruption throughout the 5 and a half week planned course. The maculopapular rash improved with the application of triamcinolone topical cream once daily for the pruritus on the affected areas, still withholding the capecitabine. Due to the limitation of other chemotherapeutic options in lieu of 5-fluorouracil (5-FU) or capecitabine-capecitabine is a prodrug that is enzymatically converted to 5-FU (2)—with this curative treatment protocol and the cutaneous-only involvement of the vasculitis, the decision was to re-challenge the patient with capecitabine with close monitoring of any systemic involvement. The capecitabine was re-introduced 10 days after the date it was initially withheld. Two days later, the patient again developed an identical maculopapular rash with similar distribution. A UA at this time was negative, excluding the possibility of renal/systemic involvement. The patient was started on prednisone 20 mg daily throughout the remaining 3 weeks of the chemoradiation treatment with the continuation of the capecitabine. Approximately 3 days from the initiation of the prednisone, the skin lesions were improving: the rash on the legs was no longer palpable and the rash on the arms was fading. Prednisone (20 mg) was continued until the last dose of capecitabine, which coincided with the last day of RT treatment. After completing chemoradiation treatment, the prednisone was tapered as follows: 15 mg daily for 5 days, followed by 10 mg daily for 5 days, 5 mg daily for 5 days, 2.5 mg daily for 5 days, and was then stopped.
Outcome and follow-up
Six weeks after the completion of the chemoradiation treatment the patient underwent a robotic abdominoperineal resection with a bloc posterior vaginectomy of the rectal tumor. Pathology reported residual, invasive, moderately-differentiated adenocarcinoma with 5-10% viable tumor and a tumor regression grade of 2 [0-3], which indicates an excellent response to the neoadjuvant chemoradiation treatment. The final pathological staging was primary tumor:ypT3, regional lymph nodes:ypN0, and distant metastases:pMX. The patient will require four months of adjuvant chemotherapy as per the current guidelines, given the high risk of recurrence with the T3 disease. The patient received 5-FU based chemotherapy in the form of 2,400 mg/m2 5-FU continuous infusion over 46 hours beginning day 1 as part of FOLFOX regimen (oxaliplatin, fluorouracil, and leucovorin) repeated every 2 weeks, as part of the standard protocol the patient received dexamethasone 20 mg intravenous in 50 mL normal saline over 15 minutes for prevention of oxaliplatin hypersensitivity, the patient completed the first cycle of the chemotherapy without any reported vasculitic rash.
Discussion
Vasculitis is a systemic disease that can be an idiopathic primary process or secondary to an underlying pathology (3). The infiltration of the blood vessel walls by activated leukocytes induces damage to the mural structures with fibrinoid necrosis which leads to loss of vessel integrity and hemorrhage with the characteristic maculopapular rash (3,4).
The current standard of treatment for locally advanced rectal cancer is chemoradiation treatment followed by surgical resection (1). This treatment improves local control of the tumour and reduces the risk of local recurrence, which is the most common recurrence pattern in rectal cancer. The treatment involves the delivery of chemotherapy using infusional 5-FU with RT over a period of 5 weeks. Current evidence supports the long-term therapeutic equivalence and non-inferiority of daily oral capecitabine compared to concomitant intravenous 5-FU during RT for neoadjuvant therapy for locally advanced rectal cancer (5,6).
Capecitabine is preferred clinically over 5-FU due to the convenience of the oral administration verses 5-FU administration, which requires intravenous or port access with continuous infusion over 46 hours with a portable infusion pump. One previous case report described 5-FU and oxaliplatin induced vasculitis in metastatic colorectal cancer treated with 5-FU, requiring switching to another chemotherapeutic agent (7). A second case report described vasculitis secondary to 5-FU and folinic acid (leucovorin) (8). To our knowledge there have been no prior reports of capecitabine induced leukocytoclastic vasculitis. None of the above reports successfully continued the 5-FU and in both cases the 5-FU was discontinued. Our decision in continuing the capecitabine in treating this patient was due to a lack of alternative chemotherapeutic options in combination with radiation therapy in the setting of locally advanced rectal cancer with curative intent. The only other alternative was 5-FU; yet capecitabine is metabolized to 5-FU and is likely to cause vasculitis, too (2), yet the use of 5-FU in the adjuvant setting in the same patient did not cause leukocytoclastic vasculitis rash, one could argue the use of the intravenous dexamethasone could have masked that.
Our case illustrates the feasibility of controlling vasculitis with oral steroid without interrupting the curative treatment protocol of locally advanced rectal cancer, which includes the culprit drug. It is paramount to be aware of the possibility of systemic involvement and to be vigilant with close monitoring of any sign that could suggest such an involvement. Fortunately, our patient did not develop any signs of systemic involvement and was regularly checked for renal involvement by UA on each clinic visit.
Learning points/take-home messages
- Capecitabine-induced vasculitis is a rare side effect of the drug.
- Drug-induced vasculitis should be considered in patients who develop a maculopapular rash while receiving capecitabine.
- Ruling out systemic involvement of vasculitis is critical in patients who develop cutaneous vasculitis.
- Capecitabine-induced vasculitis can be treated with an oral steroid without the need for discontinuation of the drug once systemic involvement has been excluded, with close monitoring of any systemic involvement.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [PubMed]
- European Medicines Agency. Capecitabine Teva: EPAR - Product Information. Teva Pharma B.V. 10 January 2014. Retrieved 11 December 2014.
- Watts RA, Scott DG. Recent developments in the classification and assessment of vasculitis. Best Pract Res Clin Rheumatol 2009;23:429-43. [PubMed]
- Langford CA. 15. Vasculitis. J Allergy Clin Immunol 2003;111:S602-12. [PubMed]
- Hofheinz RD, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 2012;13:579-88. [PubMed]
- O'Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol 2014;32:1927-34. [PubMed]
- Quack H, Erpenbeck L, Wolff HA, et al. Oxaliplatin-Induced Leukocytoclastic Vasculitis under Adjuvant Chemotherapy for Colorectal Cancer: Two Cases of a Rare Adverse Event. Case Rep Oncol 2013;6:609-15. [PubMed]
- Pellegrini F, Astorino S, Castaldi N, et al. Small vessel vasculitis related to 5-fluorouracil and folinic acid. Eur J Dermatol 2010;20:862-3. [PubMed]