Predicting complete response: is there a role for non-operative management of rectal cancer?
Introduction
Surgery has been the cornerstone in the management of patients with resectable rectal cancer. Selected patients with distal rectal, well-differentiated pT1 lesions can be treated with local excision alone with close follow-up. In patients with pT1 tumors with adverse pathologic features, and patients with pT2 tumors, two prospective trials by Radiation Therapy Oncology Group (RTOG) and Cancer and Leukemia Group B (CALGB) Intergroup demonstrated excellent local control rates and survival with local excision followed by adjuvant chemoradiation therapy (CRT) (1,2). Patients with early rectal cancers treated with pre-operative CRT followed by local excision also resulted in excellent local control. Borschitz et al. reported a long-term local recurrence rate of 7% in 237 patients with cT2-3 disease who underwent 5-fluorouracil (5-FU)-based CRT followed by local excision (3). The American College of Surgeons Oncology Group (ACOSOG) single-arm, prospective study of T2N0 rectal cancer patients who received neoadjuvant CRT and local excision demonstrated high rates of treatment response, with 34 (44%) of 77 patients experiencing a pathological complete response (pCR) (4).
In patients with more locally advanced (cT3-4) rectal cancers, pre-operative CRT has been used to downstage tumors before planned resection. The landmark German Rectal Cancer Trial randomized 823 patients with cT3-4N+ rectal cancer to either preoperative or postoperative CRT and demonstrated significantly improved local control with preoperative CRT (local recurrence rate at 5 years of 6% vs. 13% with adjuvant CRT). Among patients with low-lying tumors who were to require abdominoperineal resection, those received preoperative CRT were twice as likely to undergo a sphincter-sparing operation (5). Another randomized trial by the National Surgical Adjuvant Breast and Bowel Project (NSABP) investigated the same question but was closed early due to poor accrual. Of the 267 patients enrolled, preoperative CRT demonstrated a trend toward better disease-free survival (DFS) (6).
These studies demonstrated benefits in preoperative CRT in patients with both early and more advanced rectal cancer. It is effective in inducing tumor regression; in fact, approximately 15-27% of patients who undergo preoperative CRT experience a pCR in which no residual tumor is reported on histologic examination of total mesorectal excision (TME) (7). In a meta-analysis by Maas et al. including 3,105 patients, of which 484 patients achieved a pCR after preoperative CRT, it was shown that patients with pCR had significantly increased DFS. The 5-year crude DFS was 83% for patients with pCR and 66% for those without (7). Whether surgery and its risk of complications in these patients could have been avoided is a topic of investigation. Until recently, the only means to detect complete response reliably is through surgical resection and microscopic evaluation of the specimen. There is growing evidence that regimented clinical assessment after CRT can reliably identify patients who achieved clinical complete response (cCR), allowing avoidance of immediate surgery. We will discuss the concept of nonoperative management in patients with rectal cancer who achieved cCR after CRT in this article.
“Wait-and-see”
In 2004, Habr-Gama et al. first published their experience with 265 patients with resectable cT2-4N0/N+ rectal adenocarcinoma who underwent preoperative CRT consisting of 5,040 cGy over 6 weeks, leucovorin, and bolus 5-FU administrated intravenously for 3 consecutive days on the first and last 3 days of CRT. At 8 weeks, all patients underwent repeat evaluation, including endoscopy with biopsy. In a later report, fluorodeoxyglucose positron-emission tomography (FDG-PET)/computed tomography (CT) was also reported to be used in post-CRT assessment (8). The presence of any significant residual ulcer or positive biopsies was considered incomplete clinical response and the patient went onto TME. Patients without any abnormalities were considered to have cCR and were referred to monthly physical and digital rectal examination (DRE), proctoscopy, biopsies, and serum carcinoembryonic antigen (CEA) level testing for the first year, every 2 months in the second year, and every 6 months in the third year. Abdominal and pelvic CT scans and chest radiographs were repeated every 6 months for the first year. Of the 265 patients, 71 patients had a cCR 8 weeks after CRT and were enrolled in the wait-and-see cohort. The majority of these patients had T3 disease (69%, T2 =20%, T4 =11%) and did not have radiologic evidence of nodal metastasis (77%, N+ =23%). Among the 71 patients, the 5-year overall survival (OS) was 100% and DFS was 92%, compared with 88% and 83%, respectively, among the patients who did not achieve cCR and went onto immediate TME. Only 2 patients in the wait-and-see group developed local recurrence 56 months after CRT completion; they were salvaged by local excision and brachytherapy. These promising results led the authors to conclude that surgical resection may be safely avoided in patients appropriately identified achieving cCR after CRT (9).
Subsequent to their initial publication, the authors published several updates of their experience with patients treated with preoperative 5-FU-based CRT spanning from 1991 to 2009 (10-13). The largest series was composed of 361 patients with cT2-4 tumors and 99 (27%) achieved a sustained cCR at 12 months. Only 5 patients among the 99 cCR patients developed local recurrence. The 5-year DFS was 85% and OS was 93% (11).
In 2011, Maas et al. from the Netherlands published a prospective series of 21 patients with a cCR who were managed nonoperatively with a wait-and-see policy (14). Between 2004 and 2010, 192 patients with cT1-3N0-2 were treated with CRT consisting of 5,040 cGy over 28 fractions with concurrent capecitabine. At 6-8 weeks after CRT, magnetic resonance imaging (MRI) was performed. In addition to standard T2-weighted imaging, diffusion-weighted MRI (DWI) was used to determine the presence of residual tumoral tissue (high signal on DWI) at the primary site, and MRI enhanced with either ultra-small superparamagnetic iron oxide or gadofosveset trisodium was used to evaluate nodal status. If no residual tumor was seen on post-CRT MRI, endoscopy with biopsy was performed. A patient was only determined to achieve a cCR when no residual tumor or nodal disease was seen on MRI, no residual tumor was seen at endoscopy, negative biopsy was achieved after CRT, and there was no palpable tumor on DRE. Among the 21 patients who met this criteria, an intensive follow-up protocol was carried out, which consisted of DRE, MRI, endoscopy with biopsies, CT scan of chest and abdomen, and CEA measurements (Table 1). With a mean follow-up of 25 months, only 1 patient developed endoluminal recurrence and underwent surgical salvage. The 2-year OS in this cohort was 100% and DFS was 89%. A control cohort of 20 patients who were found to have pCR had a 2-year OS of 91% and DFS of 93%, similar to patients with cCR, and enrolled in the wait-and-see protocol (14).
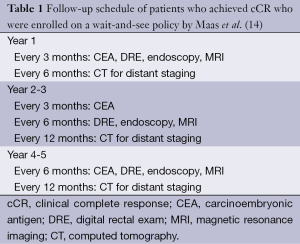
Full table
At Memorial Sloan Kettering Cancer Center, a retrospective review of patients treated between January 2006 and August 2010 compared outcomes of 32 stage I-III rectal cancer patients with a cCR to CRT who were treated nonoperatively to 57 patients with a pCR after radical rectal resection. With a median follow-up time of 28 months for the nonoperative group, 6 patients developed local recurrence and all were salvaged surgically. Three of these patients also developed distant metastases. The 2-year distant DFS and OS were similar for nonoperative and rectal resection groups (15). These studies show that, with accurate identification of patients who achieved cCR and rigorous follow-up, patients could be safely monitored without undergoing immediate TME and still have excellent oncologic outcomes. Table 2 provides a summary of the key nonoperative management studies.
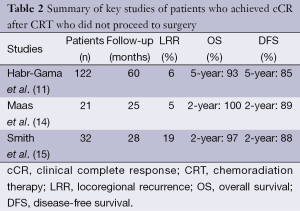
Full table
Assessment of complete clinical response
Identifying accurately patients who achieved a cCR after CRT is arguably the most important aspect of a nonoperative approach in rectal cancer management. DRE, while an important clinical practice, has been shown to be a poor method for determining cCR when used alone. In 2005, Guillem et al. evaluated DRE immediately preceding resection and found that it only identified 21% of pCR patients, thought to be due to local inflammation and fibrosis interpreted as tumor remnant (16). Endoscopy with biopsy can provide additional information to DRE; nevertheless, a negative biopsy could represent a false negative and persistent disease could not be ruled out. In a prospective study of 46 patients who were treated with preoperative CRT for rectal cancer, 22 patients underwent presurgical endoscopic biopsies. While the biopsies were negative in the 6 patients who were found to have pCR on TME, the biopsies were also negative in 11 of 16 cases with residual cancer, yielding a concordance rate of 59% between endoscopic biopsies and surgical specimens (17). Moreover, neither DRE nor endoscopy assesses for regional nodal status after CRT.
Given the limitations of DRE or endoscopy in restaging after CRT, other modalities are needed to assess for residual disease. Endorectal ultrasound (US), while useful in initial staging, has limited benefits after CRT due to the fibrotic tissue. In a large study of 235 patients comparing post-CRT endorectal US staging and pathologic staging, it was reported that endorectal US only correctly matched the T stage in 54% and N stage in 75% of patients (18). Both FDG-PET and CT scans were evaluated prospectively in a recent study by Guillem et al. in the identification of complete response after preoperative CRT (19). A total of 121 patients with rectal cancer were prospectively enrolled in the study, and both FDG-PET and CT scans were obtained before and after CRT. While 26 (21%) patients had a pCR after CRT, only 54% of the pCR patients were classified as having a cCR on preoperative PET scan, and only 19% of the patients were classified as having a cCR on preoperative CT scan. Of the pathologic incomplete responders, PET and CT scans were able to identify 66% and 95% of the patients as incomplete responders, respectively. The authors concluded that neither PET nor CT scan alone has adequate predictive value to be clinically useful in identify patients with complete response after CRT.
In 2013, van der Paardt et al. reported a meta-analysis including 33 studies and 1,556 patients on MRI imaging for restaging locally advanced rectal cancer after neoadjuvant treatments (20). For tumor stage, the authors reported a mean sensitivity of 50% and specificity of 91%. In the subgroup analysis, MRI demonstrated 19% sensitivity and 94% specificity for identifying pT0 disease. This is likely due to conventional MRI’s inability to distinguish fibrosis and residual tumor accurately. However, after incorporating functional MRI imaging results, such as DWI or dynamic contrast enhanced MRI, significant improvement in sensitivity in detecting complete tumor response after CRT was seen (84%). The specificity was 85%. Dynamic contrast enhanced MRI provides perfusion characteristics of tumor, and some parameters, such as K(trans), differ markedly between patients with cCR and the incomplete responders (21). Serial T2-weighted MRI during CRT also showed promising results in predicting for tumor pCR. Kluza et al. showed that CRT induced a significant decrease in T2-weighted signal intensity distribution of 50% in complete responder. The change in T2-weighted signal intensity resulted in high diagnostic performance for identifying complete responders with an accuracy of 92% in the 39-patients study (22). For nodal stage, MRI results in a mean sensitivity of 77% and specificity of 60% (20). With a low prevalence of involved nodes after CRT, the negative predictive value of MRI was 80-90%. Gadofosveset-enhanced MRI, used in the Dutch study, demonstrated 80% sensitivity and 97% specificity in nodal staging with experienced readers (23).
From the above studies, it is appropriate to conclude that determining cCR after CRT requires utilization of multiple methods in restaging and not a single modality alone. As demonstrated by Habr-Gama et al. and Maas et al., accurate identification of cCR is achievable with a combination of physical examination, endoscopic examination, and imaging, leading to minimal local recurrence rate with nonoperative management. With the emergence of functional MRI imaging, it is hoped there will be further improvements in our accuracy in determining a cCR to therapy.
Timing of assessment
In addition to methods of assessing cCR, another area that requires further investigation is timing of examination after preoperative CRT. The reports from Habr-Gama et al. recommended a minimum of 6-8 weeks or longer interval after CRT for assessment of cCR (24). The Dutch series evaluated response at a mean of 6.5 weeks after CRT (14). There is lack of standardization in the timing of examination. As response continues over time, it is possible that more patients with cCR can be captured with longer wait times. A recent meta-analysis of 13 trials including 3,584 patients aimed to answer the question of whether a longer interval between the end of neoadjuvant CRT and surgery is associated with a higher pCR rate. Patients were divided into two groups: patients who underwent TME shorter than 6-8 weeks after CRT vs. patients who underwent TME longer than 6-8 weeks after CRT. A longer wait interval, more than the classical 6-8 weeks, from the end of CRT was found to be associated with significantly improved pCR rate (20% vs. 14% in patients who waited <6-8 weeks, P<0.001) (25). It has been showed that delaying surgery until after 12 weeks after CRT does not negatively impact oncologic outcomes (8).
Extended chemotherapy
Studies examining new imaging modalities, such as DWI MRI, and determining the optimal clinical assessment time frames are needed. Furthermore, additional chemotherapy after CRT could be another strategy in maximizing clinical response, leading to more patients with cCR qualifying for nonoperative management. Habr-Gamma et al. enrolled 70 patients with cT2-4N0-2 rectal cancer prospectively to receive concurrent CRT followed by extended chemotherapy (5-FU/leucovorin for a total of 6 cycles every 21 days). Of the 70 patients, 47 demonstrated clinical response at 10 weeks after CRT and went on to complete extended chemotherapy. Of the 47 patients, 39 demonstrated sustained cCR for 12 months after extended chemotherapy and 4 patients developed local recurrence >12 months after chemotherapy. Overall, 35 (50%) patients never underwent surgery due to sustained cCR (26). The Timing of Rectal Cancer Response to Chemoradiation consortium conducted a prospective, multicenter, Phase II study investigating extending the interval between CRT and surgery and administering additional chemotherapy during waiting period. Sixty patients underwent TME 6 weeks after completion of 5-FU-based CRT, and 67 patients with evidence of clinical response 4 weeks after CRT received 3 additional cycles of modified FOLFOX (5-FU, leucovorin, oxaliplatin) chemotherapy followed by TME 3-5 weeks later. The pCR rate was higher in patients who received additional chemotherapy (25% vs. 18% in those who did not receive additional chemotherapy) (27). Cercek et al. showed in 2014 that induction chemotherapy, followed by CRT then surgery is another possible approach to maximize cCR. In this study, FOLFOX chemotherapy was given before CRT. Of the 49 patients who underwent TME after FOLFOX followed by CRT, 47% had tumor response >90%, including 27% of patients achieving a pCR (28).
Conclusions
Nonoperative management is an emerging trend in the treatment of rectal cancer. It has the benefits of reducing surgery-related toxicities. Modern studies with rigorous post-CRT assessments demonstrated that accurately identifying patients with cCR and avoiding/delaying surgery is feasible. Intensive follow-up regimen is needed to ensure lack of clinical progression. Despite the significant progress the field has made in moving toward nonoperative management, it continues to be an area that requires organized investigations. Developing reliable methods for repeat staging after CRT, determining the optimal time frame for maximal response assessment, and understanding the role of additional chemotherapy after CRT can all potentially allow us to capture more patients with cCR that are suitable for the wait-and-see approach, preventing overtreatment in patients with rectal cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Russell AH, Harris J, Rosenberg PJ, et al. Anal sphincter conservation for patients with adenocarcinoma of the distal rectum: long-term results of radiation therapy oncology group protocol 89-02. Int J Radiat Oncol Biol Phys 2000;46:313-22. [PubMed]
- Steele GD Jr, Herndon JE, Bleday R, et al. Sphincter-sparing treatment for distal rectal adenocarcinoma. Ann Surg Oncol 1999;6:433-41. [PubMed]
- Borschitz T, Wachtlin D, Möhler M, et al. Neoadjuvant chemoradiation and local excision for T2-3 rectal cancer. Ann Surg Oncol 2008;15:712-20. [PubMed]
- Garcia-Aguilar J, Shi Q, Thomas CR Jr, et al. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol 2012;19:384-91. [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [PubMed]
- Roh MS, Colangelo LH, O'Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124-30. [PubMed]
- Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835-44. [PubMed]
- Habr-Gama A, Perez RO, Proscurshim I, et al. Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: does delayed surgery have an impact on outcome? Int J Radiat Oncol Biol Phys 2008;71:1181-8. [PubMed]
- Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 2004;240:711-7; discussion 717-8. [PubMed]
- Habr-Gama A, Perez RO, Nadalin W, et al. Long-term results of preoperative chemoradiation for distal rectal cancer correlation between final stage and survival. J Gastrointest Surg 2005;9:90-9; discussion 99-101. [PubMed]
- Habr-Gama A, Perez RO, Proscurshim I, et al. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg 2006;10:1319-28; discussion 1328-9. [PubMed]
- Habr-Gama A. Assessment and management of the complete clinical response of rectal cancer to chemoradiotherapy. Colorectal Dis 2006;8 Suppl 3:21-4. [PubMed]
- Habr-Gama A, Perez RO, São Julião GP, et al. Nonoperative approaches to rectal cancer: a critical evaluation. Semin Radiat Oncol 2011;21:234-9. [PubMed]
- Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 2011;29:4633-40. [PubMed]
- Smith JD, Ruby JA, Goodman KA, et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg 2012;256:965-72. [PubMed]
- Guillem JG, Chessin DB, Shia J, et al. Clinical examination following preoperative chemoradiation for rectal cancer is not a reliable surrogate end point. J Clin Oncol 2005;23:3475-9. [PubMed]
- Maretto I, Pomerri F, Pucciarelli S, et al. The potential of restaging in the prediction of pathologic response after preoperative chemoradiotherapy for rectal cancer. Ann Surg Oncol 2007;14:455-61. [PubMed]
- Pastor C, Subtil JC, Sola J, et al. Accuracy of endoscopic ultrasound to assess tumor response after neoadjuvant treatment in rectal cancer: can we trust the findings? Dis Colon Rectum 2011;54:1141-6. [PubMed]
- Guillem JG, Ruby JA, Leibold T, et al. Neither FDG-PET Nor CT can distinguish between a pathological complete response and an incomplete response after neoadjuvant chemoradiation in locally advanced rectal cancer: a prospective study. Ann Surg 2013;258:289-95. [PubMed]
- van der Paardt MP, Zagers MB, Beets-Tan RG, et al. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology 2013;269:101-12. [PubMed]
- Gollub MJ, Gultekin DH, Akin O, et al. Dynamic contrast enhanced-MRI for the detection of pathological complete response to neoadjuvant chemotherapy for locally advanced rectal cancer. Eur Radiol 2012;22:821-31. [PubMed]
- Kluza E, Rozeboom ED, Maas M, et al. T2 weighted signal intensity evolution may predict pathological complete response after treatment for rectal cancer. Eur Radiol 2013;23:253-61. [PubMed]
- Lambregts DM, Beets GL, Maas M, et al. Accuracy of gadofosveset-enhanced MRI for nodal staging and restaging in rectal cancer. Ann Surg 2011;253:539-45. [PubMed]
- Habr-Gama A, Perez RO, Sabbaga J, et al. Increasing the rates of complete response to neoadjuvant chemoradiotherapy for distal rectal cancer: results of a prospective study using additional chemotherapy during the resting period. Dis Colon Rectum 2009;52:1927-34. [PubMed]
- Petrelli F, Sgroi G, Sarti E, et al. Increasing the Interval Between Neoadjuvant Chemoradiotherapy and Surgery in Rectal Cancer: A Meta-Analysis of Published Studies. Ann Surg 2013. [Epub ahead of print]. [PubMed]
- Habr-Gama A, Sabbaga J, Gama-Rodrigues J, et al. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum 2013;56:1109-17. [PubMed]
- Garcia-Aguilar J, Smith DD, Avila K, et al. Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg 2011;254:97-102. [PubMed]
- Cercek A, Goodman KA, Hajj C, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw 2014;12:513-9. [PubMed]


