Transition of a pancreatic neuroendocrine tumor from ghrelinoma to insulinoma: a case report
Case report
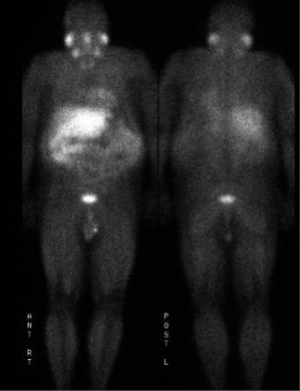
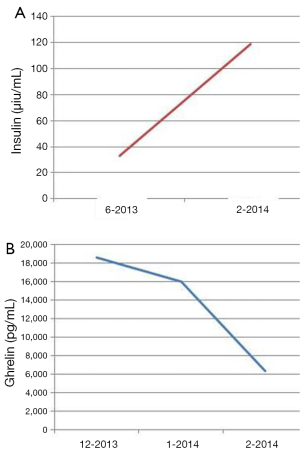
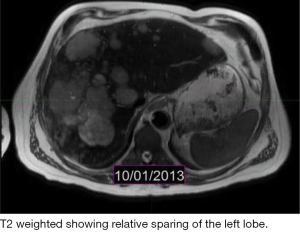
The patient is a 60-year-old Caucasian male who was diagnosed with a metastatic pancreatic neuroendocrine tumor (PNET) in December 2009 after having abdominal pain as his presenting complaint. He also complained of weight loss and fatigue. His past medical history was significant for the presence of mitral valve prolapse, type 2 diabetes mellitus and a 52 pack year of smoking history. Family history was unknown as he was adopted. Physical exam was significant only for hepatomegaly. Initial diagnostic imaging had shown tumor at the tail of the pancreas with multiple liver masses (Figure 1). Subsequent biopsy of a hepatic mass confirmed a metastatic neuroendocrine tumor (NET). His initial labs showed normal urinary 5-HIAA, glucagon, vasoactive intestinal peptide (VIP) and insulin levels. His initial chromogranin A levels were 3,625 ng/mL in December 2009 which jumped to 14,000 ng/mL in few months. He was deemed unfit for surgery due to presence of widespread metastatic disease. He was only mildly positive on a iodine-131-meta-iodobenzylguanidine (MIBG) (Figure 2), and wasn’t considered ideal candidate for MIBG therapy. He was started on Sandostatin LAR 30 mg in July 2010. At that point he complained of weight loss, flushing and intermittent severe abdominal pain but no diarrhea. He was also started on the combination of capecitabine 750 mg/m2 PO BID from day 1-14 and temozolomide 200 mg/m2 PO daily from day 10-14 in July 2013 (1). He clinically responded well to the initial three cycles of the chemotherapy with improvement in fatigue and appetite and he was able to gain nine pounds. His hepatomegaly also decreased from the level of his iliac crest to 4 cm below costal margin. His ghrelin levels progressively went down from 18,651 pg/mL in December 2013 to 16,047 pg/mL in 1/2014 and 6,342 pg/mL in 2/2014. Meanwhile his insulin level went from 33 µiu/mL in June 2013 to 119 µiu/mL in February 2014 (Figure 3). C-Peptide level was 10 ng/mL. He was symptomatically hypoglycemic and his endocrinologist started continuous glucose monitoring and increased his caloric intake. His lowest measured glucose was 36 ng/dL. Unfortunately patient passed away at home a week after his last follow up (August 2014). Immediate cause of death was not known. Though he had an extensive metastatic disease, hypoglycemia cannot be ruled out as a possible cause of death.
Discussion
PNET are rare with an incidence of 1 in 100,000 populations (2). PNETs can present either as functional or non-functional tumors. In functional tumors, the symptoms are a result of hormones such as insulin, gastrin, glucagon and VIP or others. Non-functioning tumors are often detected at late stages because they do not have a related hormone syndrome. Signs and symptoms of non-functional tumors are secondary to the tumor burden (mass effect vs. metastatic disease) (3). Ghrelin is a 28 amino acid peptide discovered in 1999 and is thought to be involved in various physiologic and pathologic processes (4). Due to relatively recent discovery of this hormone, its functions in normal homeostasis and its association with various pathologic processes are still being uncovered. Ghrelin is thought to be involved in regulation of appetite, growth hormone regulation, gastric secretion and gut motility (5). Ghrelin is primarily produced in stomach by oxyntic cells in the gastric mucosa but it has also been found in hypothalamus and other endocrine glands like pituitary and pancreas (6). Fasting induces secretion of ghrelin (7) whereas there is a sharp decline in ghrelin level postprandially (8). This indicates a role of ghrelin in regulating food intake.
The association of ghrelin with NETs has been studied by few. Corbetta et al. described the first PNET case with elevated ghrelin levels (9). Their case did not show signs or symptoms of acromegaly. Tsolakis et al. presented a case of malignant NET of stomach with hyperghrelinemia (10). Their patient however had BMI of 32 and ended up developing diabetes with HbA1c of 12.8%. On the contrary our patient went from being a diabetic to hyperinsulinemic. The association of elevated ghrelin levels with GI NETs and the clinical implication needs to be studied in detail. Our case adds to the limited literature on the natural history of gastrointestinal NETs with ghrelinemia.
Conclusions
Here, we present a case of metastatic functional PNET that initially presented with marked elevation of ghrelin, suggestive of ghrelinoma, which later transformed into a clinically significant insulinoma. PNETs are a rare entity and the natural history of PNETS with excessive ghrelin secretion is not well known. This case gives us a glimpse into an unusual variant of metastatic PNET. It also tells us that changes in functional tumor biology can sometimes be more morbid than the physical manifestations of the metastatic disease itself.
Acknowledgements
Disclosure: Dr. Ramirez is a consultant for Biotheranostics. None of other co-authors have any financial or commercial disclosure pertaining to current study.
References
- Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011;117:268-75. [PubMed]
- Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci 2004;1014:13-27. [PubMed]
- Vagefi PA, Razo O, Deshpande V, et al. Evolving patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms: the Massachusetts General Hospital experience from 1977 to 2005. Arch Surg 2007;142:347-54. [PubMed]
- Vu JP, Wang HS, Germano PM, et al. Ghrelin in neuroendocrine tumors. Peptides 2011;32:2340-7. [PubMed]
- Cheung CK, Wu JC. Role of ghrelin in the pathophysiology of gastrointestinal disease. Gut Liver 2013;7:505-12. [PubMed]
- Bordi C, D’Adda T, Azzoni C, et al. Hypergastrinemia and gastric enterochromaffin-like cells. Am J Surg Pathol 1995;19 Suppl 1:S8-19. [PubMed]
- Cummings DE, Purnell JQ, Frayo RS, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001;50:1714-9. [PubMed]
- Tschöp M, Wawarta R, Riepl RL, et al. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest 2001;24:RC19-21. [PubMed]
- Corbetta S, Peracchi M, Cappiello V, et al. Circulating ghrelin levels in patients with pancreatic and gastrointestinal neuroendocrine tumors: identification of one pancreatic ghrelinoma. J Clin Endocrinol Metab 2003;88:3117-20. [PubMed]
- Tsolakis AV, Portela-Gomes GM, Stridsberg M, et al. Malignant gastric ghrelinoma with hyperghrelinemia. J Clin Endocrinol Metab 2004;89:3739-44. [PubMed]