Surgical management of gastric cancer: the East vs. West perspective
Introduction
The global incidence, screening policies, pathology, management and outcomes of gastric cancer vary significantly by geography, especially between the East and West. While the incidence in the United States (U.S.) is estimated at 21,600 new cases a year (1), the incidence in South Korea, the country with the highest rate in the world, is 33,000 per year, a large number compared to the much smaller size of its population, followed by Mongolia, Japan and China (2,3). Because of the higher incidence in the East, South Korea and Japan, for example, have initiated a screening program for its citizens in an effort to increase rates of early detection. In fact, such systematic efforts in the East have been found to be cost-effective and have resulted in improved gastric cancer survival (4). Meanwhile, in the West, where the per capita incidence of gastric cancer is far lower, such systematic gastric cancer screening efforts for the entire population has no proven benefit.
There are, however, notable differences between the East and West. The high rate of Barrett’s esophagus in the U.S., for example, confers an increased risk of esophageal adenocarcinoma, including gastroesophageal junction tumors, which in the East are often classified as gastric cancer. Because the classification of gastroesophageal junction tumors is a controversial topic, this review will focus only on gastric cancer. The typical patient profile between the East and West differs significantly, guiding the corresponding systematic approaches to gastric cancer. Many of these differences are thought to be due to epidemiologic and environmental risk factors. Even within the U.S. population, patients of Asian descent have been found to have a higher relative overall survival compared with their counterparts of Caucasian, African-American and Hispanic descent (5-7). These inherent differences contribute to the treatment approaches adopted, which have geographic variance. Here, we review the differences in pathology, surgical and systemic therapy, and outcomes between the East and West.
Biology
One of the primary differences between the East and West to consider is gastric cancer pathology. Classically, consideration begins with anatomic localization, a factor that guides treatment and correlates with outcomes. From epidemiologic studies, gastric cancer in the West is more commonly located in the proximal stomach and presents at a more advanced stage and has a worse prognosis than in the East, where distal gastric cancers are more common (8). Additionally, lower esophageal and proximal gastric adenocarcinoma has been steadily increasing, a phenomena not observed in the East; this has been postulated to be due to a lower incidence of reflux esophagitis and Barretts metaplasia (8).
In the West, the incidence of the diffuse and signet ring histologic subtypes occurs more commonly than in the East and are associated with worse prognoses. In addition to the differences in histology, patients in the West tend to present with more advanced disease, whereas nearly half of patients in South Korea and Japan present with early stage disease, a result likely attributable to the national screening programs in those countries. Patients in the U.S. also generally have greater co-morbidities than do patients in the East (9). For example, U.S. gastric cancer patients typically present later in life and have a higher body mass index, factors linked to an increased risk of post-operative complications, all of which are critical to note when comparing outcomes in gastric cancer treatment (9).
Although the etiology and pathogenesis of gastric cancer is an avid topic of investigation without a proven definitive mechanism to date, there are many well described risk factors (10-13). Medical conditions with a known association with gastric cancer include Helicobacter pylori (H. pylori) gastric infection, chronic atrophic gastritis, intestinal metaplasia, pernicious anemia, gastric adenomatous polyps, and giant hypertrophic gastritis (Ménétrier disease) (10-12). Interestingly, in the U.S., male gender, African American race, low socioeconomic status, obesity, occupational hazards in metal and rubber work, mining, wood and asbestos dust exposure, and cigarette smoking are associated with gastric cancer, while chronic H. pylori exposure and diet have been associated with gastric cancer in the East (10-13). It has also been postulated that in U.S. Caucasian and Hispanic patients, there may be a link between Epstein-Barr virus infection (13). A high salt diet, smoked foods, nitrates, nitrites, poorly preserved foods and secondary amines are thought to alter the gastric milieu, resulting in production of N-nitroso compounds which are carcinogenic, factors which are thought to be critical to the increased incidence of gastric cancer in the East (10-13). The role of H. pylori infection in the pathogenesis of gastric cancer is a broad and controversial topic beyond the scope of this review, with multiple efforts underway to determine the exact mechanism of this link and what the implications may be for public health efforts in eradicating the organism.
Endoscopic resection
For advanced gastric cancer and most early-stage gastric cancer, gastrectomy with D2 lymphadenectomy (resection of perigastric lymph nodes and nodes along the named branches of the celiac axis) is considered standard surgical therapy. However, with advancement in techniques for local evaluation of gastric tumors with endoscopic ultrasound, as well as endoscopic resection techniques, endoscopic submucosal dissection (ESD) has become well-recognized as a treatment for early gastric cancers that are at low risk for lymph node metastases. Initial indications for endoscopic resection for early gastric cancer was differentiated histology, <2 cm in diameter, lack of ulceration or scarring, mucosal involvement only, with no lymphatic or vascular involvement (14). More recently, extended indications for ESD are differentiated tumors, without evidence of venous or lymphatic involvement, <3 cm in diameter, and confined to the mucosa or submucosa (15). Expanded criteria to include undifferentiated tumors has yielded excellent long-term survival rates (16,17); ESD is now considered a therapy that could be offered to patients who have early gastric cancer, particularly those limited to the mucosa, without adverse histologic features. Caution must be exercised for tumors with submucosal involvement due to the increased risk for occult lymph node metastases. Lymph node metastases may be present in as many as 20% of patients with early stage gastric cancer, particularly in those patients with lymphovascular invasion and larger tumor size (≥2 cm) (18). Therefore, in patients with submucosal disease, gastrectomy with associated lymphadenectomy should be considered standard of care. For patients at high-risk for surgery, ESD can be considered an option.
D1 vs. D2 lymphadenectomy
Surgery is the mainstay treatment for early stage gastric cancer and is paramount for achieving cure in patients with gastric adenocarcinoma. Barring an early T1a or in situ tumor, gastrectomy including resection of the regional lymph nodes remains the standard surgical procedure. The extent of lymphadenectomy, however, has been a greatly debated topic of controversy throughout the last few decades. The majority of Japanese and Korean (i.e., Eastern) surgeons would agree that an extended lymphadenectomy (D2) leads to improved outcomes and survival. Certainly, multiple large retrospective studies from those groups have illustrated an impressive overall survival that has not been replicated in Western series (19,20).
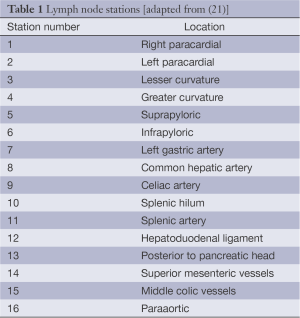
The Japanese Gastric Cancer Association (JGCA) published guidelines for surgical treatment and pathologic evaluation that grouped the perigastric and distant draining lymph nodes into 16 stations (Figure 1, Table 1) (21). These stations were then categorized into 4 levels (N1 to 4) based on the likely lymphatic drainage from the respective primary tumor location (22). The nodes along the lesser [stations 1, 3, 5] and greater [2, 4, 6] curvatures are included in the perigastric lymph node level (N1). The more distant draining lymph node stations follow the left gastric artery [7], common hepatic artery [8], celiac artery [9], splenic hilum and artery [stations 10 and 11] and are grouped in the N2 level. The most distant, or para-aortic, nodes (N3 or N4) are usually considered distant metastatic disease and are not traditionally included with gastric resections. However, these four categorization levels have recently been abandoned to prevent confusion with the TNM staging systems.
The extent of lymphadenectomy is dependent on the extent of gastrectomy being performed (i.e., total, subtotal/distal, or proximal gastrectomy) (23). For example, historically, a D2 dissection for a total gastrectomy would involve retrieval of lymph node stations 1-12 with a concomitant distal pancreatectomy and splenectomy while a D1 dissection would only require the perigastric nodes at stations 1-7. More recently, proponents have advocated a modified approach to a D2 dissection by sparing the spleen and pancreas unless directly involved with the primary tumor. This approach of sparing the pancreas and spleen has shown adequate retrieval of lymph nodes without the morbidity associated with multi-visceral resection (24,25).
A recent retrospective study evaluating 1,377 patients from the Surveillance, Epidemiology, and End-Results (SEER) database looked at the impact of the number of nodes examined and its relationship with survival as a surrogate for accurate staging (26). Total lymph node count and number of positive lymph nodes were two of the independent factors associated with survival. Significant survival benefit was observed for patients who had more than 15 N2 nodes and 20 N3 nodes examined. Although there is no consensus on the level of dissection required (D1 vs. D2) in the U.S., pathologic assessment of at least 15 nodes is considered standard of care, and D2 lymphadenectomy is recommended (27).
Japanese and South Korean surgeons routinely perform D2 lymphadenectomy for patients with gastric adenocarcinoma. The surgeon will then meticulously dissect out each lymph node station prior to sending tissue for pathologic evaluation, unlike in the U.S., where surgeons submit the gastrectomy specimen en bloc with the lymphadenectomy. Based on the extensive gastric cancer database of 3,843 patients from the experience by the National Cancer Center in Japan, the Maruyama index (MI) was created in order to create estimates for the likelihood of metastases for each lymph node station not removed by the surgeon. The index is based on 8 variables: age, sex, Borrmann classification, depth of invasion, diameter, location, position and histology (28). Studies of gastric cancer patients undergoing gastrectomy with a MI <5 versus those ≥5, had an improved median overall and relapse-free survival on univariate and multivariate analysis (29,30). Due to the complexity, however, it is infrequently utilized in the West.
Western proponents for a limited D1 resection cite two large randomized controlled trials published in the 1990s from the Netherlands and United Kingdom that were unable to show a survival benefit with extended lymphadenectomy (Table 2). The Dutch Gastric Cancer Group Trial randomized 711 patients undergoing surgery for curative intent to either D1 or D2 lymphadenectomy in 80 centers throughout the Netherlands (32). Participating surgeons were provided an instruction booklet and videotape on how to perform D2 lymphadenectomy, and an experienced Japanese gastric cancer surgeon was present for the first 6 months of the study for instruction. Patients undergoing D2 resections were more likely to have a higher operative mortality (10% vs. 4%, P=0.004) and morbidity (43% vs. 25%, P<0.001). Mature, 15-year follow-up data showed no overall survival benefit with a D2 lymphadenectomy (41). A subset analysis, however, showed a lower locoregional recurrence rate and fewer gastric cancer related deaths with D2 lymphadenectomy. Similar to the Dutch trial, the United Kingdom Medical Research Council (MRC) Gastric Cancer Surgical Trial (ST01) randomized 400 gastric adenocarcinoma patients to D1 or D2 lymphadenectomy (34). The operating surgeons were provided with a booklet and instructional video to ensure standardization of the two procedures. Again, this Western study demonstrated higher post-operative mortality (13% vs. 6.5%, P=0.04) and morbidity rates (46% vs. 28%, P<0.01) in the D2 lymphadenectomy group as well as a higher chance of undergoing concomitant pancreatectomy and splenectomy. Most notably was the significantly higher rate of anastomotic complications in the D2 dissection group, also including severe pancreatitis, pancreatic fistula, and gastric remnant necrosis. Long-term results showed no difference in overall survival, gastric cancer related deaths, or recurrence-free survival.
Full table
These trials may now be less relevant as more recent studies have shown that routine resection of the spleen and pancreatic tail for middle and proximal gastric tumors increases morbidity and perioperative mortality without long term overall survival benefit. The traditional D2 resection involves a distal pancreatectomy and splenectomy for all tumors except in the antral location, in order to adequately resect lymph node stations 10 and 11 surrounding the splenic artery and hilum. In the UK MRC trial, subset analysis of patients undergoing pancreatico-splenectomy, splenectomy alone, or preservation of both organs showed survival difference, with the poorest survival in those undergoing multi-visceral resection (35). Similarly, the Dutch trial performed a multivariate analysis and showed increased mortality associated with splenic or pancreatic resections. This likely contributed to the lack of survival difference between D1 and D2 resections.
More recently, however, studies from the East and West have shown improved morbidity and mortality with avoidance of routine splenectomy and pancreatectomy compared to traditional D2 resection (42-44). The Italian Gastric Cancer Study group randomized 267 patients with gastric adenocarcinoma to a D1 or modified D2 resection (39). Routine splenectomy and pancreatectomy were not performed unless direct extension by the primary tumor (T4) was noted. No statistically significant difference was noted between the groups in regards to morbidity or in-hospital mortality. Due to this most recent data, surgeons in the Eastern hemisphere are routinely adopting a modified technique for D2 resections and preserving the pancreas and spleen.
The difference in survival and results between Eastern and Western surgeons is likely multi-factorial. Some have pointed to the theory of stage migration as the etiology for improved survival with D2 resection with Eastern surgeons. With an extended lymphadenectomy, a greater number of lymph nodes are retrieved with a higher chance of detecting a positive node. A recent retrospective analysis of 79 patients undergoing D2 vs. D1 lymphadenectomy from Kaiser Permanente Los Angeles showed a significantly greater number of nodes retrieved with a D2 lymphadenectomy (mean, 26 vs. 9 nodes, P<0.0001) (45). Within the D2 lymphadenectomy group, 39% showed additional lymph node metastases in the extended portion of the dissection, altering 16% of the TNM staging. Additional lymph node dissection beyond a D2 is traditionally not recommended. A prospective trial spearheaded by the Japanese Clinical Oncology Group randomized 523 patients with gastric cancer to D2 or D2 plus para-aortic lymph node dissection (38). Although, as expected, the operative time and estimated blood loss were increased with the extended dissection, the overall and recurrence-free survival showed no significant difference.
Minimally invasive approaches
Since the first minimally invasive distal gastrectomy for gastric cancer was described by Kitano et al. (46) in 1994, there have been multiple studies comparing this to the classic open approach. The theoretical benefits of faster recovery time, decreased operative blood loss and lower morbidity rates with minimally invasive gastrectomy are weighed against the concern for oncologic safety with adequate lymphadenectomy for accurate staging.
There are several randomized clinical trials (RCT) (47-51), predominantly out of the East, and the Korean Laparoscopic Gastrointestinal Surgery Study Group (KLASS trial) has published the largest trial to date (48). This group randomized 342 patients with early gastric cancer (limited to T1N0, T1N1, or T2N0 by preoperative staging) to laparoscopic or open distal gastrectomy. The authors showed no difference in post-operative morbidity and mortality between the groups. In regards to oncologic safety, the authors did not evaluate the number of nodes removed between the two groups. However, there was no statistically significant difference in terms of the rate of D1 vs. D2 dissection done between the two groups. No long-term results regarding locoregional recurrence or overall survival are currently available.
Due to the small number of randomized clinical trials and the low number of patients in each study, a recent meta-analysis by Vinuela et al. (52) included several high quality non-randomized studies (NRCT) comparing laparoscopic and open distal gastrectomy for gastric cancer. Twenty-five studies (including 6 RCTs and 19 NRCTs) were included for a total of 3,055 patients from both the Eastern and Western hemispheres. Although laparoscopic distal gastrectomies were associated with longer operative times, estimated blood loss was lower, and a decreased length of hospital stay and overall complication rate was demonstrated. There was no difference between groups with respect to in-hospital mortality. Open gastrectomy, however, showed a significantly higher number of lymph nodes retrieved compared to the laparoscopic approach. However, the proportion of patients with less than the 15 nodes was similar in each group. The potential effect on long-term survival with laparoscopic gastrectomy is still unclear. Additionally, since the majority of studies are predominantly focused on early stage disease, a study bias may be present, and it remains to be seen whether minimally-invasive gastrectomy is an effective approach for more advanced stages, especially as seen in the Western hemisphere.
Gastric cancer patients who have a proximal or bulky tumor are not candidates for a distal/subtotal gastrectomy and should be considered for a total gastrectomy. Laparoscopic total gastrectomy is considered technically more difficult than its distal gastrectomy counterpart and, therefore, less widely practiced. However, increasing experience with minimally invasive techniques and better instrumentation has prompted more utilization for gastric cancer patients. A recent meta-analysis looking at 15 NRCT comparing laparoscopic and open total gastrectomies was published (53). Similar to the data for laparoscopic distal gastrectomies, laparoscopic total gastrectomy was associated with lower estimated blood loss and complication rate, although with a longer operative time. No difference in mortality was noted. In addition, oncologic resections were similar, as there was no significant difference in the number of nodes retrieved between the groups. Unfortunately, no data on long-term survival was available. Minimally-invasive gastrectomy, either laparoscopic or robotic, is currently regarded as an approach that should be offered by experienced surgeons who are familiar with these techniques.
Cytoreductive surgery and heated intraperitoneal chemotherapy (HIPEC)
The presence of peritoneal disease is classified as stage IV disease; however, recent data suggests that these patients should not necessarily be precluded from surgical resection. Cytoreduction with the administration of HIPEC has long been advocated for treatment of peritoneal malignancies related to appendiceal cancer, mesothelioma, and more recently, colon adenocarcinoma and gastric adenocarcinoma. Yang et al. published results of a randomized phase III trial in which 68 patients with peritoneal carcinomatosis were randomized to either cytoreductive surgery or cytoreductive surgery with HIPEC using intraperitoneal Cisplatin and Mitomycin C. The rate of adverse events was noted to be similar between groups, and the cytoreductive surgery plus HIPEC patients had an improved overall survival, median of 11 months versus 6.5 with cytoreductive surgery alone (P=0.046) (54). The University of Pittsburgh evaluated 23 patients with gastric peritoneal carcinomatosis who underwent cytoreductive surgery with HIPEC using Mitomycin C and observed major morbidity in over 50% of the cohort and a median overall survival of 9.5 months, concluding that cytoreductive surgery with HIPEC may offer survival benefit in a carefully selected population (55).
More aggressive chemotherapy regimens have been recently advocated, with the institution of bidirectional chemotherapy: systemic chemotherapy in addition to the intraperitoneal chemotherapy. Yonemura recently published results from a specialized peritoneal malignancy center in Japan, evaluating 194 patients treated with bidirectional therapy: intraperitoneal docetaxel and cisplatin and oral S-1 for four cycles. Patients who responded to this therapy were then taken for cytoreductive surgery and HIPEC with docetaxel (56). Major complications were observed in 23.6% and an improved medial survival of 15.8 months was noted. A meta-analysis of 20 randomized controlled trials using various intraperitoneal agents also demonstrated that HIPEC conferred a 2-year survival advantage (57).
More recently, Rudloff et al. randomized 17 metastatic gastric cancer (including those with liver and lung disease) patients to cytoreductive surgery with HIPEC and systemic chemotherapy (FOLFOXIRI: 5-FU, leucovorin, oxaliplatin, and irinotecan) vs. systemic chemotherapy alone (58). The median overall survival for the HIPEC group was 11.3 months compared to 4.3 months in the systemic chemotherapy only arm. However, definitive conclusions on the superiority of HIPEC with systemic chemotherapy should be deferred since this study was limited by small numbers of patients.
While cytoreductive surgery and HIPEC is not commonly considered for patients with gastric carcinomatosis, patients with a low peritoneal carcinoma index may have an improved survival with this treatment modality. While most surgeons advocate initial treatment with systemic therapy, patients with stable disease, low-volume peritoneal disease and good functional status may be considered for this treatment modality. Caution for enthusiasm regarding cytoreductive surgery and HIPEC must be exercised until future research can further clarify the optimal treatment and timing for this diverse population of metastatic gastric cancer patients. The survival of patients receiving systemic therapy only reported in these trials falls short of survival of metastatic patients previously reported in other, larger studies. Therefore, HIPEC should be performed on protocol at institutions that routinely perform HIPEC, in select patients who have demonstrated stability of disease and survival on standard chemotherapies.
Outcomes
Although both the East and West utilize the American Joint Committee on Cancer (AJCC) staging system for determination of prognosis, relative survival differs markedly even when matched by stage. For example, when comparing Korean and U.S. high-volume centers, disease specific survival after R0 resection was greater in Korea, with a 5-year gastric-cancer-related probability of death of 17% versus 32% in the U.S (59). Interestingly, a subset analysis of a T1N0 cohort at the same institutions demonstrated no difference in rates of death due to gastric cancer (60). A meta-analysis addressing this question, comparing published disease specific survival rates in randomized control trials, demonstrated improved relative 5-year survival in the East with an adjusted odds ratio of 3.22 (95% confidence interval: 1.85-5.58) (61). These results were demonstrated even after adjusting for patient age, chemotherapy, gender, and tumor size, factors historically attributed as reasons for differences in survival outcomes between East and West.
Other than the differences in surgical treatment as discussed above, there are also important differences between East and West in perioperative therapy to consider. Lesions T2 or greater, or with evidence of lymph node disease, are typically treated first with systemic therapy in the West, unlike in the East where surgical resection is typically performed, even for advanced gastric cancer (23,62). Theoretical advantages for pre-operative therapy include: demonstration of an in vivo response to therapy, treatment of occult micrometastatic disease, better health of patients who may subsequently receive the full chemotherapy regimen, and increased likelihood of margin-negative surgical resection of tumor.
The British medical research council adjuvant gastric cancer infusional chemotherapy (MAGIC) trial introduced neoadjuvant chemotherapy as standard of care in the West. The trial demonstrated that patients with operable gastric, esophageal, and gastroesophageal cancer had improved survival when treated with preoperative and postoperative chemotherapy, 23% with surgery-alone versus 36% with surgery and chemotherapy (63). In addition, the authors illustrated a higher curative resection rate (79% vs. 70%, P=0.03) for patients who underwent neoadjuvant therapy. This increase in curative resection rate (R0 resection) for neoadjuvant therapy is mirrored in other studies as well (64,65). While this approach reflects the treatment philosophy in the West, in the East the results were criticized because of the inclusion of esophageal cancers and the limited extent of lymphadenectomy in surgical treatment. It should be noted, however, that phase II and phase III trials of preoperative S-1 and cisplatin in Japanese series, including the extended lymphadenectomy, demonstrated improved survival compared to historical controls (66,67). For patients with bulky nodal or para-aortic nodal disease, improved overall survival was also observed when randomized to neoadjuvant S-1 and cisplatin followed by surgery with an extended lymphadenectomy, but further trials are under way (67,68).
Conclusions
Although etiologic and pathologic differences exist in the presentation of gastric cancer treated in the West versus the East, surgical techniques developed in countries of high-incidence have become more universal. It is widely accepted that gastrectomy with a modified D2 lymphadenectomy (sparing the distal pancreas and spleen) confers adequate staging information, with the goal of obtaining a minimum of 15 lymph nodes. As minimally-invasive techniques continue to be developed, oncologic safety and equivalence to the standard open gastrectomy remains to be seen. With better efficacy of systemic chemotherapy, more aggressive approaches to surgical resection, including cytoreduction and HIPEC, can also be considered in selected patients. These techniques appear to be applicable to patients in both the Eastern and Western hemispheres.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- American Cancer Society. Cancer Facts & Figures 2013. Atlanta: American Cancer Society, 2013.
- Jung KW, Won YJ, Kong HJ, et al. Prediction of cancer incidence and mortality in Korea, 2013. Cancer Res Treat 2013;45:15-21. [PubMed]
- GLOBOCAN 2008 Database; Available online: http://globocan.iarc.fr
- Cho E, Kang MH, Choi KS, et al. Cost-effectiveness outcomes of the national gastric cancer screening program in South Korea. Asian Pac J Cancer Prev 2013;14:2533-40. [PubMed]
- Kunz PL, Gubens M, Fisher GA, et al. Long-term survivors of gastric cancer: a California population-based study. J Clin Oncol 2012;30:3507-15. [PubMed]
- Kim J, Sun CL, Mailey B, et al. Race and ethnicity correlate with survival in patients with gastric adenocarcinoma. Ann Oncol 2010;21:152-60. [PubMed]
- Al-Refaie WB, Tseng JF, Gay G, et al. The impact of ethnicity on the presentation and prognosis of patients with gastric adenocarcinoma. Results from the National Cancer Data Base. Cancer 2008;113:461-9. [PubMed]
- Davis PA, Sano T. The difference in gastric cancer between Japan, USA and Europe: what are the facts? what are the suggestions? Crit Rev Oncol Hematol 2001;40:77-94. [PubMed]
- Bickenbach KA, Denton B, Gonen M, et al. Impact of obesity on perioperative complications and long-term survival of patients with gastric cancer. Ann Surg Oncol 2013;20:780-7. [PubMed]
- Kurtz RC, Sherlock P. The diagnosis of gastric cancer. Semin Oncol 1985;12:11-8. [PubMed]
- Scheiman JM, Cutler AF. Helicobacter pylori and gastric cancer. Am J Med 1999;106:222-6. [PubMed]
- Fenoglio-Preiser CM, Noffsinger AE, Belli J, et al. Pathologic and phenotypic features of gastric cancer. Semin Oncol 1996;23:292-306. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Ahn JY, Jung HY. Long-term outcome of extended endoscopic submucosal dissection for early gastric cancer with differentiated histology. Clin Endosc 2013;46:463-6. [PubMed]
- Soetikno R, Kaltenbach T, Yeh R, et al. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol 2005;23:4490-8. [PubMed]
- Abe S, Oda I, Suzuki H, et al. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy 2013;45:703-7. [PubMed]
- Oda I, Oyama T, Abe S, et al. Preliminary results of multicenter questionnaire study on long-term outcomes of curative endoscopic submucosal dissection for early gastric cancer. Dig Endosc 2014;26:214-9. [PubMed]
- An JY, Baik YH, Choi MG, et al. Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: analysis of a single institutional experience. Ann Surg 2007;246:749-53. [PubMed]
- Nakajima T, Nishi M. Surgery and adjuvant chemotherapy for gastric cancer. Hepatogastroenterology 1989;36:79-85. [PubMed]
- Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg 1987;11:418-25. [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. [PubMed]
- Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Jpn J Surg 1981;11:127-39. [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. [PubMed]
- Furukawa H, Hiratsuka M, Ishikawa O, et al. Total gastrectomy with dissection of lymph nodes along the splenic artery: a pancreas-preserving method. Ann Surg Oncol 2000;7:669-73. [PubMed]
- Zhang CH, Zhan WH, He YL, et al. Spleen preservation in radical surgery for gastric cardia cancer. Ann Surg Oncol 2007;14:1312-9. [PubMed]
- Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol 2007;14:317-28. [PubMed]
- Kwon SJ. Evaluation of the 7th UICC TNM Staging System of Gastric Cancer. J Gastric Cancer 2011;11:78-85. [PubMed]
- Kampschöer GH, Maruyama K, van de Velde CJ, et al. Computer analysis in making preoperative decisions: a rational approach to lymph node dissection in gastric cancer patients. Br J Surg 1989;76:905-8. [PubMed]
- Hundahl SA, Macdonald JS, Benedetti J, et al. Surgical treatment variation in a prospective, randomized trial of chemoradiotherapy in gastric cancer: the effect of undertreatment. Ann Surg Oncol 2002;9:278-86. [PubMed]
- Peeters KC, Hundahl SA, Kranenbarg EK, et al. Low Maruyama index surgery for gastric cancer: blinded reanalysis of the Dutch D1-D2 trial. World J Surg 2005;29:1576-84. [PubMed]
- Dent DM, Madden MV, Price SK. Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg 1988;75:110-2. [PubMed]
- Bonenkamp JJ, Songun I, Hermans J, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet 1995;345:745-8. [PubMed]
- Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999;340:908-14. [PubMed]
- Cuschieri A, Fayers P, Fielding J, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet 1996;347:995-9. [PubMed]
- Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 1999;79:1522-30. [PubMed]
- Wu CW, Hsiung CA, Lo SS, et al. Randomized clinical trial of morbidity after D1 and D3 surgery for gastric cancer. Br J Surg 2004;91:283-7. [PubMed]
- Wu CW, Hsiung CA, Lo SS, et al. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol 2006;7:309-15. [PubMed]
- Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 2008;359:453-62. [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg 2010;97:643-9. [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014;101:23-31. [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [PubMed]
- Maruyama K, Sasako M, Kinoshita T, et al. Pancreas-preserving total gastrectomy for proximal gastric cancer. World J Surg 1995;19:532-6. [PubMed]
- Biffi R, Chiappa A, Luca F, et al. Extended lymph node dissection without routine spleno-pancreatectomy for treatment of gastric cancer: low morbidity and mortality rates in a single center series of 250 patients. J Surg Oncol 2006;93:394-400. [PubMed]
- Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg 2006;93:559-63. [PubMed]
- Putchakayala K, Difronzo LA. D2 lymph node dissection improves staging in patients with gastric adenocarcinoma. Am Surg 2011;77:1326-9. [PubMed]
- Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146-8. [PubMed]
- Huscher CG, Mingoli A, Sgarzini G, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 2005;241:232-7. [PubMed]
- Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 2010;251:417-20. [PubMed]
- Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc 2005;19:168-73. [PubMed]
- Kitano S, Shiraishi N, Fujii K, et al. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery 2002;131:S306-11. [PubMed]
- Hayashi H, Ochiai T, Shimada H, et al. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc 2005;19:1172-6. [PubMed]
- Viñuela EF, Gonen M, Brennan MF, et al. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg 2012;255:446-56. [PubMed]
- Xiong JJ, Nunes QM, Huang W, et al. Laparoscopic vs open total gastrectomy for gastric cancer: a meta-analysis. World J Gastroenterol 2013;19:8114-32. [PubMed]
- Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575-81. [PubMed]
- Magge D, Zenati M, Mavanur A, et al. Aggressive locoregional surgical therapy for gastric peritoneal carcinomatosis. Ann Surg Oncol 2014;21:1448-55. [PubMed]
- Canbay E, Mizumoto A, Ichinose M, et al. Outcome data of patients with peritoneal carcinomatosis from gastric origin treated by a strategy of bidirectional chemotherapy prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a single specialized center in Japan. Ann Surg Oncol 2014;21:1147-52. [PubMed]
- Coccolini F, Cotte E, Glehen O, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol 2014;40:12-26. [PubMed]
- Rudloff U, Langan RC, Mullinax JE, et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol 2014;110:275-84. [PubMed]
- Strong VE, Song KY, Park CH, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg 2010;251:640-6. [PubMed]
- Strong VE, Song KY, Park CH, et al. Comparison of disease-specific survival in the United States and Korea after resection for early-stage node-negative gastric carcinoma. J Surg Oncol 2013;107:634-40. [PubMed]
- Markar SR, Karthikesalingam A, Jackson D, et al. Long-term survival after gastrectomy for cancer in randomized, controlled oncological trials: comparison between West and East. Ann Surg Oncol 2013;20:2328-38. [PubMed]
- Waddell T, Verheij M, Allum W, et al. Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi57-63. [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [PubMed]
- Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 2010;28:5210-8. [PubMed]
- Iwasaki Y, Sasako M, Yamamoto S, et al. Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol 2013;107:741-5. [PubMed]
- Fujitani K. Overview of adjuvant and neoadjuvant therapy for resectable gastric cancer in the East. Dig Surg 2013;30:119-29. [PubMed]
- Yoshikawa T, Nakamura K, Tsuburaya A, et al. A phase II study of preoperative chemotherapy with S-1 (S) and cisplatin (P) followed by D3 gastrectomy for gastric cancer with extensive lymph node metastasis (ELM): survival results of JCOG 0405. J Clin Oncol 2011;29:abstr 70.