Patterns of failure for stage I ampulla of Vater adenocarcinoma: a single institutional experience
Background
Ampullary adenocarcinoma is a rare cancer that accounts for less than 1% of all gastrointestinal malignancies (1). Given the location of these tumors, patients often present with relatively early stage disease with symptoms related to biliary obstruction. Given higher potential for surgical resection compared with other hepatobiliary tumors, prognosis is favorable, with multiple studies demonstrating 5-year overall survival (OS) rates ranging from 30% to 60% (2-8). Risk factors adversely impacting prognosis include positive surgical margins, nodal involvement, and tumor differentiation and size (9-11).
The primary treatment modality for ampullary carcinomas is pancreaticoduodenectomy. Despite the relatively favorable prognosis for ampullary tumors, patients with high-risk features often develop locoregional and distant recurrence (12). Due to the rarity of this disease, the benefits of adjuvant therapy have primarily been analyzed through retrospective and institutional series, and the role of adjuvant therapy remains unclear.
Many authors recommend the use of adjuvant chemoradiotherapy (CRT) for patients with nodal involvement, involved margins and advanced tumor stage (8-11,13). Published series advocate the use of adjuvant therapy in advanced tumors (i.e., T3/T4 and node positive disease) based on high locoregional recurrence and low OS rates reported in these patients. With surgery alone, 5-year local control (LC) rates of 33-47% have been reported (8-11). However, most investigators have not advised adjuvant therapy for T1-T2N0 tumors (stage I) given the more favorable 5-year OS rates ranging from 40-100% (7,8,10,11,14). However, contemporary population-based data from the National Cancer Data Base (Commission on Cancer of the American College of Surgeons and the American Cancer Society) demonstrated poor outcomes with 5-year OS rates of 40-44% for T1-T2N0 tumors (15). We hypothesize that even early stage (T1-T2N0) ampullary tumors may have failure rates high enough to warrant adjuvant therapy. We undertook this study to evaluate patterns of failure and disease-related outcomes for patients with T1-T2N0 tumors undergoing pancreaticoduodenectomy, with or without adjuvant CRT.
Methods
This study was approved by the Duke University Institutional Review Board. The records of all patients evaluated between 1976 and 2011 who were diagnosed with stage I (T1-T2, node negative) ampullary carcinoma were reviewed. Ampullary carcinoma was defined as tumors arising in the ampulla or major papilla. Patients with tumors of the duodenum, bile duct, pancreas or minor papillae on pathologic examination were excluded. Patients who presented with disease metastasis or those who received neoadjuvant CRT were excluded as were patients with positive resection margins. Surgical pathology was staged according to the American Joint Committee on Cancer Guidelines, 7th edition (15).
Surgery
All patients underwent pancreaticoduodenectomy with curative intent. Prior to surgery, patients were evaluated by cross-sectional imaging with abdominal and pelvic computed tomography (CT) or magnetic resonance imaging (MRI), as well as endoscopic ultrasonography (EUS) and endoscopic retrograde cholangiopancreatography (ERCP). Patients’ pathology, including histological stage and grade, as well as perineural (PNI) and lymphovascular (LVI) invasion and margin involvement, were abstracted. For patients whose original pathology did not include information regarding PNI or LVI, the pathology slides were reexamined by the Duke University Pathology department. Both diagnostic biopsy and Whipple specimens were reviewed in some instances given that some stage I tumors were resected at biopsy with minimal or no residual tumor in the Whipple specimen.
Chemoradiotherapy (CRT)
The decision to deliver adjuvant therapy was based on physicians’ preference. Multi-field external beam radiation therapy was used to treat the tumor bed and locoregional lymph nodes, including pancreaticoduodenal, superior mesenteric artery, celiac, and porta hepatis nodal regions. Patients were treated in 1.8 Gy fractions, 5 days consecutively per week. Prior to 1997, radiation plans used 2-dimensional anterior-posterior/posterior-anterior with opposed lateral beams; following 1997, patients underwent 3-dimensional treatment planning. Concurrent chemotherapy regimen was determined by the treating Medical Oncologist and was fluoropyrimidine-based in all cases.
Statistical analysis
All statistical analyses were performed by Duke Cancer Institute’s statistics department. Time-to-event endpoints were estimated using the Kaplan–Meier method, calculated from the time of surgery. P values were calculated using the Log-Rank method. LC, disease-free survival (DFS), metastasis-free survival (MFS) and OS were measured. Local failure was defined as recurrence in the initial tumor bed or locoregional lymph node basins. Recurrences outside these regions were designated as distant failures. Patients without local failure were censored either at the time of distant failure, time of death or, if alive, at the last follow-up. DFS was defined as the time to the first instance of a local or distant failure, and was censored at last follow-up if alive or at death if there was no evidence of recurrence. Similarly, MFS was defined as the time to distant failure and was censored at last follow-up if alive or at death if there was no evidence of distant failure. OS was defined as the time between surgery and death and was censored at the last follow-up for patients alive at the time of analysis. Patients returned to clinic for follow-up approximately every 3 months following treatment. Patterns of failure were assessed during follow-up primarily through radiographic imaging with biopsy of the suspected recurrent disease when clinically appropriate. Median follow-up time was calculated using the reverse Kaplan–Meier method (16).
Results
Forty-four patients diagnosed with stage I ampullary cancer underwent pancreaticoduodenectomy. Median patient age at diagnosis was 65 (range, 38-79). Patients often presented with obstructive symptoms including abdominal pain, jaundice as well as pancreatitis. Of these patients, 31 were treated with surgery alone, while 13 received surgery and adjuvant CRT. There were no cases of perioperative deaths. Median radiation dose was 4,500 cGy (range, 3,060-5,040 cGy) and chemotherapy was fluorouracil-based (infusional 5-fluorouracil or capecitabine). Seventeen patients had T1 tumors while 27 were found to have T2 disease. The median follow-up time for all patients was 8.0 years. Patient characteristics are summarized in Table 1. Patients returned to clinic every 3-6 months with physical examination and CT following treatment completion. Those who demonstrated no evidence of disease after 5 years were then frequently followed by their local physician. The patients that received adjuvant therapy did not exhibit statistically significant differences in age, tumor grade or stage versus surgery-alone patients.
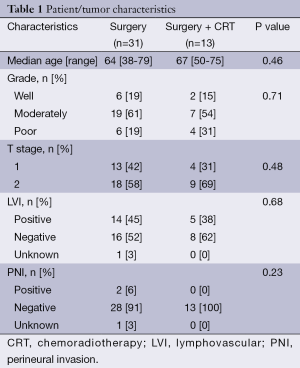
Full table
Of the 44 patients, 14 patients (32%) experienced disease recurrence with some component of locoregional failure. Eleven (79%) of these patients demonstrated both local and distant failures. Of these, nine (82%) demonstrated local and distant disease diagnosed synchronously. Of the two patients whose local and distant recurrences were diagnosed metachronously, one exhibited local failure first while the other was diagnosed with distant disease initially. Of the distant failures, 10 patients (91%) developed liver metastases and one patient (9%) lung metastases.
Of the 31 patients undergoing surgery only, 12 patients (39%) developed recurrent disease. Two patients (17%) demonstrated local recurrence only, while 10 (83%) were found to have both local and distant recurrence. Half of these patients initially presented with T1 disease while the other half T2. Six of these patients (50%) had no other adverse pathologic factors, 5 (42%) were found to have LVI, and 1 (8%) to have PNI. Two (15%) of 13 patients receiving adjuvant therapy subsequently experienced disease recurrence. Both patients presented with T2 disease without any other adverse pathologic features and were diagnosed with synchronous local and distant recurrence.
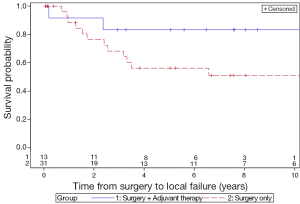
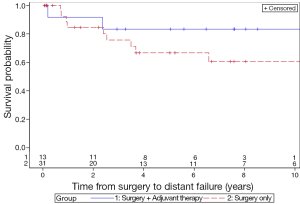
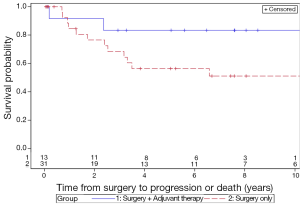
The 5-year LC rates for patients treated with surgery only and surgery with adjuvant therapy were 56.3% and 83.3%, respectively (P=0.13) (Figure 1). The 5-year MFS rates for the surgery and adjuvant group were 66.8% and 83.3%, respectively (P=0.31) (Figure 2). The 5-year DFS rates for the surgery only and surgery with adjuvant therapy groups were 56.4% and 83.3%, respectively (P=0.13) (Figure 3). The 5-year OS rates for the surgery only and surgery with adjuvant therapy groups were 53.4% and 68.4%, respectively (P=0.09) (Figure 4), with corresponding 10-year OS rates of 28.8% and 68.4%, respectively. There were no differences observed in disease-related outcomes based on T staging, tumor histology or PNI/LVI.
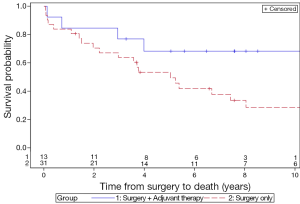
Discussion
Ampullary cancer is a rare malignancy which tends to have better prognosis than pancreatic adenocarcinomas (17,18). Willett et al. demonstrated 5-year OS of 55% for “high risk” disease (≥T3, poorly differentiated histologic findings, involved surgical margins or lymph nodes) and 80% survival for “low risk” disease (T1/T2 tumors without high risk features) (10). Similarly, several subsequent retrospective series reported similar survival, with 5-year OS of small patient subsets with T1-T2 disease, ranging from 40% to 100% (Table 2) (7,8,11,13,14). However, it is important to note that almost all of these studies included lymph node positive patients, with authors reporting outcomes based solely on T-staging without indication of nodal status or other adverse histologic features for these particular subsets. Despite the inclusion of these patients, the favorable outcomes relative to more advanced disease prompted the authors to recommend adjuvant therapy only for advanced tumors or select high risk features (10).
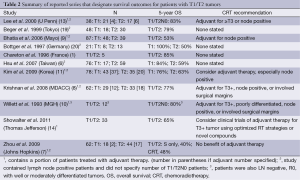
Full table
Based on the results of these studies, adjuvant CRT has not been usually recommended for patients with T1/T2N0 disease (8,10,13). However, updated data from the National Cancer Data Base (Commission on Cancer of the American College of Surgeons and the American Cancer Society) from 1998-2002 reported 5-year OS of only 40% and 44% for T1N0 and T2N0 tumors, respectively (15). In addition, a recent randomized European study suggested a possible survival benefit for chemotherapy versus observation following surgery for periampullary tumors, although specific impact on patterns of relapse were not described (21). While this possible benefit was seen on secondary endpoint multivariate analysis (correcting for prognostic variables), approximately 47% of patients in this trial were diagnosed with T1/T2 tumors, and 41% were lymph node negative, although specific analysis of stage I patients was not pursued. The poor disease-related outcomes may potentially be explained by persistent locoregional/nodal disease outside the surgical resection field as demonstrated by Palta et al. (5). In the present analysis of early stage patients from a larger group of patients, we hypothesized that adjuvant CRT may lead to improvements in LC and possibly OS, even in patients with relatively early stage tumors.
Our study demonstrated a surprisingly high locoregional failure rate (39%) for the surgery only cohort compared to patients receiving combined modality therapy (15%) for these early stage patients. Similarly, we witnessed trends towards higher MFS and OS in the patients treated with adjuvant CRT. While not statistically significant, given small number of patients treated with combined modality, our data suggests potential benefit from adjuvant CRT. Zhou et al., demonstrated 5-year OS of 40% and 48% for T1/2 tumors treated with surgery only and combined modality therapy, respectively, and recommended no adjuvant treatment based on the negligible survival improvement from additional therapy (7). However, the median follow-up time in this study was 19.3 months, compared with 8.0 years in this series, and patients with nodal involvement were also included in their analysis. We believe that the long-term follow-up and exclusion of node positive patients in our study allows a more accurate assessment of possible benefit of CRT in patients with early stage ampullary cancers.
Given that 39% of patients who received surgery alone experienced locoregional failure, we believe there is a clinically significant risk of residual subclinical disease following radical resection, even for early tumors. These findings are consistent with a previous study suggesting that local resection for early ampullary tumors is inadequate in preventing disease recurrence (22). In our series, the addition of adjuvant CRT appeared to reduce local failure rates, although not statistically significant, given small patient numbers. Additionally, there appeared to be a large number of patients who developed distant metastases, most commonly in the liver. Given most distant metastases occurred synchronously with local recurrence, it is possible that these tumors were more biologically aggressive or that the development of locoregional failure facilitates distant metastases development (23-25). This concept suggests that improved LC, through addition of radiation therapy, may potentially prevent both local and subsequent distant recurrences. It is also likely that the ability to detect local recurrences with contemporary imaging techniques is suboptimal, likely underestimating local recurrence rates, notably given that local recurrences may be overlooked once distant metastases have developed. Surprisingly, we did not notice any significant differences in disease outcomes based on tumor size nor pathologic grade. Additionally, while almost half of the patients presented with LVI, this did not appear to have any effect on disease-related outcomes on outcomes analysis.
Our study is limited by several factors, including its retrospective nature, relatively small patient numbers and small number of patients receiving adjuvant treatment. Consequently, although the survival curves depicted differences at the 5- and especially 10-year time points, our outcomes only trended towards significance due to limited power. Given the rarity of this disease and lack of randomized prospective studies evaluating adjuvant CRT, these analyses remain instructive. To our knowledge, this is the largest reported non-population-based series specifically reporting outcomes for patients with stage I (T1/T2N0) ampullary cancers, notably those receiving adjuvant CRT.
Conclusions
Our data suggest that LC and OS for stage I ampullary carcinomas may not be as favorable as previously described. Patients who receive adjuvant CRT may derive a benefit in LC and potentially other disease-related outcomes. Based on our experience, we recommend adjuvant CRT for selected patients with resected stage I ampullary carcinoma.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Chareton B, Coiffic J, Landen S, et al. Diagnosis and therapy for ampullary tumors: 63 cases. World J Surg 1996;20:707-12. [PubMed]
- Matory YL, Gaynor J, Brennan M. Carcinoma of the ampulla of Vater. Surg Gynecol Obstet 1993;177:366-70. [PubMed]
- Monson JR, Donohue JH, McEntee GP, et al. Radical resection for carcinoma of the ampulla of Vater. Arch Surg 1991;126:353-7. [PubMed]
- Talamini MA, Moesinger RC, Pitt HA, et al. Adenocarcinoma of the ampulla of Vater. A 28-year experience. Ann Surg 1997;225:590-9; discussion 599-600. [PubMed]
- Palta M, Patel P, Broadwater G, et al. Carcinoma of the ampulla of Vater: patterns of failure following resection and benefit of chemoradiotherapy. Ann Surg Oncol 2012;19:1535-40. [PubMed]
- Hsu HP, Yang TM, Hsieh YH, et al. Predictors for patterns of failure after pancreaticoduodenectomy in ampullary cancer. Ann Surg Oncol 2007;14:50-60. [PubMed]
- Zhou J, Hsu CC, Winter JM, et al. Adjuvant chemoradiation versus surgery alone for adenocarcinoma of the ampulla of Vater. Radiother Oncol 2009;92:244-8. [PubMed]
- Krishnan S, Rana V, Evans DB, et al. Role of adjuvant chemoradiation therapy in adenocarcinomas of the ampulla of vater. Int J Radiat Oncol Biol Phys 2008;70:735-43. [PubMed]
- Bhatia S, Miller RC, Haddock MG, et al. Adjuvant therapy for ampullary carcinomas: the Mayo Clinic experience. Int J Radiat Oncol Biol Phys 2006;66:514-9. [PubMed]
- Willett CG, Warshaw AL, Convery K, et al. Patterns of failure after pancreaticoduodenectomy for ampullary carcinoma. Surg Gynecol Obstet 1993;176:33-8. [PubMed]
- Kim K, Chie EK, Jang JY, et al. Role of adjuvant chemoradiotherapy for ampulla of Vater cancer. Int J Radiat Oncol Biol Phys 2009;75:436-41. [PubMed]
- Kopelson G, Galdabini J, Warshaw AL, et al. Patterns of failure after curative surgery for extra-hepatic biliary tract carcinoma: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys 1981;7:413-7. [PubMed]
- Lee JH, Whittington R, Williams NN, et al. Outcome of pancreaticoduodenectomy and impact of adjuvant therapy for ampullary carcinomas. Int J Radiat Oncol Biol Phys 2000;47:945-53. [PubMed]
- Showalter TN, Zhan T, Anne PR, et al. The influence of prognostic factors and adjuvant chemoradiation on survival after pancreaticoduodenectomy for ampullary carcinoma. J Gastrointest Surg 2011;15:1411-6. [PubMed]
- Edge S, Byrd DR, Compton CC, et al. eds. AJCC Cancer Staging Handbook.Manual, 7th ed. New York: Springer, 2009:235-8.
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343-6. [PubMed]
- Branum GD, Pappas TN, Meyers WC. The management of tumors of the ampulla of Vater by local resection. Ann Surg 1996;224:621-7. [PubMed]
- Howe JR, Klimstra DS, Moccia RD, et al. Factors predictive of survival in ampullary carcinoma. Ann Surg 1998;228:87-94. [PubMed]
- Beger HG, Treitschke F, Gansauge F, et al. Tumor of the ampulla of Vater: experience with local or radical resection in 171 consecutively treated patients. Arch Surg 1999;134:526-32. [PubMed]
- Böttger TC, Boddin J, Heintz A, et al. Clinicopathologic study for the assessment of resection for ampullary carcinoma. World J Surg 1997;21:379-83. [PubMed]
- Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA 2012;308:147-56. [PubMed]
- Zhong J, Palta M, Willett CG, et al. The role of local excision in invasive adenocarcinoma of the ampulla of Vater. J Gastrointest Oncol 2013;4:8-13. [PubMed]
- Gnerlich JL, Luka SR, Deshpande AD, et al. Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch Surg 2012;147:753-60. [PubMed]
- Wright JL, Patil SM, Temple LK, et al. Squamous cell carcinoma of the anal canal: patterns and predictors of failure and implications for intensity-modulated radiation treatment planning. Int J Radiat Oncol Biol Phys 2010;78:1064-72. [PubMed]
- Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys 1982;8:1-11. [PubMed]


