A 26-year-old female with metastatic primary gastrointestinal malignancy presenting as menorrhagia
Introduction
Krukenberg tumor is a rare metastatic ovarian carcinoma with a usual underlying gastrointestinal primary tumor, the commonest being gastric cancer (1). The presentation is similar to other ovarian tumors, often with vague symptoms of abdominal pain and distension, menstrual cycle changes and dyspareunia in a young patient. Occasionally, the primary tumor may manifest with life threatening complications such as in our case.
Case report
A 26-year-old Hispanic gravida 4, para 3 female presented to the gynecology clinic at our institution for heavy vaginal bleeding for the preceding 3 months. The patient also reported irregular menstrual cycles, bilateral pelvic pain, dysuria and suprapubic discomfort with urination for 3 months. Her pelvic examination revealed a firm fixed cervix with bilateral enlarged ovaries. A transvaginal ultrasound showed bilateral enlarged ovaries, right measuring 6.21 cm and left measuring 7.28 cm, irregularly shaped with free fluid in cul-de-sac. Computed tomography (CT) scan of the abdomen and pelvis showed severe bilateral hydronephrosis, worse on the right and enlarged uterus of mixed density. Large heterogeneous mass is noted in the pelvis that appeared bi-lobed measuring 6.7 cm on right and 7.8 cm on left. Positron emission tomography (PET) scan revealed a small bladder nodule measuring 2.3 cm × 1.8 cm, small amount of free fluid in the pelvis and adjacent to the liver, severe right-sided hydronephrosis and hydroureter, bilateral moderate to severely enlarged ovaries. PET scan official reading did not mention any abnormal activity in the stomach. She underwent bilateral ureteral stents placement. At that time, bladder, endometrial and endocervical biopsies were obtained.
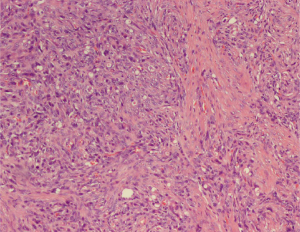
Pathology from the cervical biopsy revealed fragments infiltrated by malignant neoplasm, with a differential diagnosis that included carcinosarcoma. The tissue specimen was send to a tertiary institution for a second opinion and was reported as “cervical mucosa with diffuse infiltration by spindle and epithelioid appearing cells with hyperchromatic and pleomorphic round and spindle nuclei”. Immunostaining revealed diffuse positivity for pancytokeratin, SMA, vimentin and focal positivity for CD10. The tissue staining was negative for P63, CK5/6, and calretinin. These findings were supportive of a likely diagnosis of carcinosarcoma. She underwent exploratory laparotomy with radical hysterectomy, bilateral salpingo-oophorectomy, omentectomy, lymph node dissection, tumor debulking and partial cystectomy with bladder repair. The tissue specimen revealed extensive malignant neoplasm in right (8 cm) and left (9 cm) ovaries, fallopian tubes, myometrium and cervix. The pathological specimen was reported as poorly differentiated tumor with extensive lymphovascular and perineural invasion with the largest tumor burden noted in the ovaries (Figure 1). Immunostaining showed diffuse positivity for AE1/3 and smooth muscle actin (AMA), and focal positivity for inhibin. Differential diagnosis included carcinosarcoma. The pathologic specimen was send to another tertiary care center for second opinion.
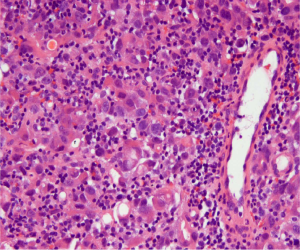
The patient returned to our emergency department within a week after her discharge, with multiple episodes of hematemesis. Shortly after her presentation, the results of pathological examination of the specimen from her debulking surgery were received, and showed Krukenberg tumor, with primary site being indeterminate. An esophagogastroduodenoscopy (EGD) showed a single, five centimeter, broad based, friable mass along the greater curvature of the stomach, three centimeters below the gastro-esophageal junction. The mass was found to be actively bleeding. The bleeding was controlled by embolization of the left gastric and left gastroepiploic arteries. Tissue biopsy of the gastric mass revealed poorly differentiated adenocarcinoma, diffuse type, with signet ring cells, identified as the primary source of the Krukenberg tumor (Figure 2). Immunostaining was positive for cytokeratin AE1/AE3 and negative for HER2/neu and CD 20. HER-2 fluorescence in situ hybridization was positive. She was started on adjuvant chemotherapy with folinic acid, 5-fluorouracil, and oxaliplatin (FOLFOX) a month later. The patient underwent gastrectomy with heated intraperitoneal chemotherapy 3 months later with subsequent removal of the left ureteral stent 2 months later. A follow-up PET scan showed no residual or recurrent neoplasm and residual left sided hydronephrosis. The patient completed 12 cycles of FOLFOX chemotherapy without major side effects. PET/CT scan done 1 month after the completion of chemotherapy showed left-sided pelvic activity that was felt to be associated with the bowel. The patient underwent colonoscopy one year after her initial presentation. This revealed an inflammatory mass in the rectum. Biopsy revealed poorly differentiated adenocarcinoma; a subset of cells showed signet ring cell morphology, similar to the prior studies confirming metastatic tumor in the rectum. The patient underwent low anterior resection with re-anastomosis. A repeat PET/CT scan now showed a new focus of increased metabolic activity within the vaginal cuff concerning for tumor recurrence with a plan for surgery, along with administration of adjuvant herceptin.
Discussion
Krukenberg tumor is commonly defined as an ovarian carcinoma that contains a significant component of mucin-filled signet-ring cells lying within a cellular stroma of ovarian origin, accounting for 1% to 1.5% of all ovarian tumors (1). Krukenberg tumor is primarily seen in the young, with the average age ranging from 40 to 46 years (2). Recent case reviews have reported that 35-45% of patients are younger than 40 years of age, similar to our patient. With the young age at presentation, an association with pregnancy is not uncommon and may present a diagnostic challenge (2,3).
Nearly all Krukenberg tumors are considered to be metastatic, with rare cases labeled as primary tumors. The existence of the latter has been challenged in recent literature and is thought to be the result of occult primary tumors (4). The most common sites of origin include the stomach (76%), colon (11%), breast cancer (4%), biliary system (3%) and the cecal appendix (3%). A minority (3%) come from the pancreas, cervix, bladder and renal pelvis (5). In our case, on re-review of the initial PET scan with the radiologist, an FDG-avid mass was found in the stomach. Given the rarity of the tumor, it was misread as normal gastric activity. While the exact mode of transmission remains to be elucidated (6), it is widely accepted that the most likely routes are lymphatic, hematogenous and peritoneal spread. Early lymphatic invasion followed by subsequent spread into the systemic circulation, is postulated as the predominant metastatic pathway. The close relation of the retroperitoneal lymph nodes draining the upper abdominal organs, with lymphatic vessels from the ovary is often thought to account for the frequent bilateral involvement (up to 80% at time of diagnosis) by the tumor (6). In context of the hematogenous route, it has been suggested that the relatively early age at diagnosis is a result of the high ovarian vascularity, facilitating vascular metastasis (7). Peritoneal spread has not been shown as a predominant mode for metastasis to the ovaries with the near universal absence of evidence of peritoneal involvement such as seeding, adhesions, implantations, or tumor infiltration on the external ovarian surface.
On gross pathology, these tumors often present bilaterally as asymmetrically enlarged ovaries with a bosselated surface. The capsular surface is typically smooth and devoid of implants (7). Cut sections show yellow or white, hard, solid masses ranging from just a few to more than twenty centimeters across, which may have grayish-red gelatinous areas of cystic degeneration (5,7). Light microscopy reveals a diffuse infiltration of a stroma made of large spindle shaped cells by mucin laden ‘signet ring’ cells with eccentric hyperchromatic nuclei. Evidence of stromal edema forming pseudocysts or an intense desmoplastic response may be noted as well (7). The signet ring cells may occur singly or in nests, clusters, tubules, acini, trabeculae or cords, often many of these in the same tumor (4). As a result, the histology does not always correspond to that of the primary tumor.
Presenting symptoms are often vague and include abdominal pain and distension, menstrual cycle changes and dyspareunia, as in our patient. Virilization may be a presenting feature in some (8). Ascites is a late feature, but is noted in up to half of those diagnosed (5). The Pseudo-Meig syndrome of accompanying right hydrothorax may be rarely seen (4).
Krukenberg tumors are usually suspected with a CT scan showing solid ovarian tumors with well demarcated cystic lesions, often with strongly contrast enhancing walls (9). However, diagnosis relies on the characteristic histology with identification of intra-cytoplasmic mucin in the signet ring cells. Immunohistochemistry is often helpful in distinguishing between a primary ovarian tumor and a metastatic tumor, and also between different primary sites of origin (Table 1) (11).
Most patients with Krukenberg tumors die within a year of the diagnosis (5). Very rarely, longer survival of up to 7 years has been described (12,13). Prognostic factors are not yet well established. The prognosis is poor when the primary tumor is identified after the ovarian metastasis and even poorer if there is no primary identified (14). CA-125 levels have also been used for prognostication. Kikkawa and his colleagues found that the 5-year survival rate was lower in patients in whom preoperative serum CA-125 levels were greater than 75 U/mL compared with those with levels less than that (14). Our patient’s initial CA-125 was 275 U/mL, decreasing to 107 U/mL after 6 months, following surgery and 11 cycles of FOLFOX.
The optimal treatment strategy remains unclear. Surgical resection of metastatic ovarian tumor has been associated with improved survival in patients with metachronous Krukenberg tumor from gastric cancer in the absence of distant metastasis other than that to the ovaries (15). Advanced gastric cancer which has invaded the gastric serosa carries a very poor prognosis, as peritoneal dissemination frequently occurs even after curative surgical resection (16). To date, various attempts have been made to treat peritoneal dissemination of gastric cancer, including aggressive surgery (17), peritonectomy for cytoreduction, intraperitoneal chemotherapy and/or hyperthermia, and systemic chemotherapy (18-21). However, contributions of these therapies to patient survival have been unsatisfactory. Recent clinical trials have revealed that chemotherapy with the anticancer agent S-1, which is composed of FT (tegafur), CDHP (5-chloro-2,4-dihydroxypyridine, which inhibits the 5-FU degradation enzyme dihydropyrimidine dehydrogenase), and Oxo (otastat potassium, which reduces 5-FU gastrointestinal toxicity) might be a promising therapy for patients with advanced gastric cancer (10).
Conclusions
Krukenberg tumor like other ovarian tumors presents with vague symptoms but at times presents with manifestations of the primary tumor, most often a gastric cancer. Treatment is largely surgical, with removal of both the tumor as also any identified primary. Prognosis is exceedingly poor, and a combination of novel chemotherapeutic and biological agents along with surgery may lead to better outcomes, although data on this is lacking.
Acknowledgements
We gratefully acknowledge Dr. Edward J. Tanner for his expert assistance.
Disclosure: The authors declare no conflict of interest.
References
- Scully RE, Young RH, Clement PB. Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament. In: Rosai J, Sobin LH. eds. Atlas of Tumor Pathology, 3rd series, fascicle 23. Washington, DC: Armed Forces Institute of Pathology, 1995.
- Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol 2006;30:277-99. [PubMed]
- Byun S, Kim HK, Kim GW, et al. Bone metastasis with paraplesia of Krukenberg tumor in pregnancy. Korean J Obstet Gynecol 2012;55:830-3.
- Al-Agha OM, Nicastri AD. An in-depth look at Krukenberg tumor: an overview. Arch Pathol Lab Med 2006;130:1725-30. [PubMed]
- Gómez Zuleta M, Benito LF, Almonacid C. Friedrich Krukenberg of Krukenberg’s Tumor: Report of a series of cases. Rev Col Gastroenterol 2012;27:119-24.
- Israel SL, Helsel EV Jr, Hausman DH. The challenge of metastatic ovarian carcinoma. Am J Obstet Gynecol 1965;93:1094-101. [PubMed]
- Young Robert H. From Krukenberg to today: the ever present problems posed by metastatic tumors in the ovary: part I. Historical perspective, general principles, mucinous tumors including the Krukenberg tumor. Adv Anat Pathol 2006;13:205-27. [PubMed]
- Ances IG, Ganis FM. Metabolism of testosterone by virilizing Krukenberg tumor of the ovary. Am J Obstet Gynecol 1968;100:1062-70. [PubMed]
- Megibow AJ, Hulnick DH, Bosniak MA, et al. Ovarian metastases: computed tomographic appearances. Radiology 1985;156:161-4. [PubMed]
- Koizumi W, Kurihara M, Nakano S, et al. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology 2000;58:191-7. [PubMed]
- Chu PG, Weiss LM. Immunohistochemical characterization of signet-ring cell carcinomas of the stomach, breast, and colon. Am J Clin Pathol 2004;121:884-92. [PubMed]
- Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol 2006;30:277-99. [PubMed]
- McGoogan LS, Hatch KD. Krukenberg tumor--report of two cases. Nebr Med J 1972;57:409-15. [PubMed]
- Kikkawa F, Shibata K, Ino K, et al. Preoperative findings in non-gynecologic carcinomas metastasizing to the ovaries. Gynecol Obstet Invest 2002;54:221-7. [PubMed]
- Cheong JH, Hyung WJ, Chen J, et al. Survival benefit of metastasectomy for Krukenberg tumors from gastric cancer. Gynecol Oncol 2004;94:477-82. [PubMed]
- Mori T, Fujiwara Y, Yano M, et al. Prevention of peritoneal metastasis of human gastric cancer cells in nude mice by S-1, a novel oral derivative of 5-Fluorouracil. Oncology 2003;64:176-82. [PubMed]
- Fujimura T, Yonemura Y, Nakagawara H, et al. Subtotal peritonectomy with chemohyperthermic peritoneal perfusion for peritonitis carcinomatosa in gastrointestinal cancer. Oncol Rep 2000;7:809-14. [PubMed]
- Yu W, Whang I, Suh I, et al. Prospective randomized trial of early postoperative intraperitoneal chemotherapy as an adjuvant to resectable gastric cancer. Ann Surg 1998;228:347-54. [PubMed]
- Nakajima T, Nishi M, Kajitani T. Improvement in treatment results of gastric cancer with surgery and chemotherapy: experience of 9,700 cases in the Cancer Institute Hospital, Tokyo. Semin Surg Oncol 1991;7:365-72. [PubMed]
- Yonemura Y, Bando E, Kawamura T, et al. Cytoreduction and intraperitoneal chemotherapy for carcinomatosis from gastric cancer. Cancer Treat Res 2007;134:357-73. [PubMed]
- Yonemura Y, Kawamura T, Bandou E, et al. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg 2005;92:370-5. [PubMed]