The emerging role of neoadjuvant chemotherapy for rectal cancer
Introduction
In 2014 it is estimated that there will be more than 136,000 new cases of colorectal cancer diagnosed as well as greater than 50,000 colorectal cancer associated deaths in the United States. Approximately 40,000 patients will be diagnosed with rectal cancer (1). National uptake of screening via colonoscopy has markedly increased in the last decade, with a corresponding decrease in the incidence of colorectal cancer over this time. In contrast, among individuals under the age of 50, a slight rise in the rates of distal colon and rectal cancers has been observed in the US, as recently reported in Norway (2). Over the last three decades, outcomes of patients with rectal cancer have substantially improved stage for stage, likely attributable to improvements in therapy (3). Prior to the standard use of radiotherapy, systemic therapy, and transmesorectal excision (TME) surgery, both local and distant recurrences represented major problems in the treatment of rectal cancer. Unacceptably high rates of devastating local recurrences prompted multiple efforts to improve local control. In the ensuing years, the benefit of peri-operative radiotherapy, specifically 5-FU based chemoradiation, was established to improve outcomes in patients with rectal cancer (4-7). The primary benefit seen is in reduced local recurrence rates, with a less consistent impact on disease free and overall survival. Moreover, this benefit is demonstrated to be greater with the use of pre-operative rather than post-operative chemoradiation (4). This has led to the incorporation of neoadjuvant 5-FU-based chemoradiation into the standard treatment paradigm for locally advanced rectal cancer.
Notably, since the initial trials of chemoradiation, surgical approaches for rectal cancer evolved significantly, with TME becoming the standard of care. This technique involves en bloc removal of the mesorectum, including the primary tumor and the associated perirectal lymph nodes via meticulous dissection so as not to disrupt the mesorectal plane. The advent of TME brought single-institution reports of local recurrence rates as low as 4-9%, compared with rates of 32-35% through use of conventional surgery (8). Of course, these vast surgically mediated improvements in local control brought into question by some the necessity of pre-operative radiotherapy; as noted, the benefit most consistently observed with chemoradiation has been the reduction in local recurrence rates. For the most part, the pivotal trials evaluating the benefit of adding of radiotherapy to surgery incorporated a suboptimal, but formerly standard non-TME surgical approach. However, the Dutch neoadjuvant trial of short course pre-operative radiotherapy (5×5 Gy) utilized the modern surgical approach, TME, and yet demonstrated a consistent benefit of improved local control (9). Outcomes appear comparable with the two techniques: short course pre-operative radiotherapy and 5-FU based neoadjuvant chemoradiation, though the former has not been widely adopted in the US to date (10,11). Given the bulk of the data supporting pre-operative chemoradiation, as well as demonstration of improved outcomes with TME, utilization of both modalities is currently the standard approach for locally advanced rectal cancer (T3, T4 or node positive disease). Most guidelines also support the addition of post-operative adjuvant chemotherapy, which is administered for the majority of patients (12).
While the data for adjuvant chemotherapy in rectal cancer treated via multimodality therapy is less robust, it is generally accepted that adjuvant chemotherapy is a necessary part of therapy. GITSG protocol 7175 closed early following interim analysis and demonstrated improvements in recurrence rates and disease free survival (DFS) with the use of adjuvant chemotherapy, with or without radiotherapy (13). A survival benefit was not established here. However, the subsequently published NSABP R-01 study, utilizing adjuvant 5-FU based chemotherapy (5-FU, semustine, and vincristine), and the NCCTG study which added 5-FU and methyl-CCNU to radiotherapy both demonstrated that post-operative chemotherapy improves survival (14,15). Of course, refinements in these regimens followed. These chemotherapy choices do not represent the standard for colorectal cancer today. Through investigation, the options of infusional 5-FU or bolus 5-FU and leucovorin were established as the optimal regimens (16,17). The non-inferiority of capecitabine was subsequently confirmed (18). Further building upon this, the MOSAIC trial and NSABP C-07 demonstrated an additional improvement in DFS with the addition of oxaliplatin to 5-FU based adjuvant therapy for colon cancer (19,20). This has led to the routine offering of 5-FU based chemotherapy, typically FOLFOX to stage III and high risk stage II colon cancer patients. A Cochrane meta-analysis of 21 randomized controlled trials supports this practice in rectal cancer, demonstrating a 25% reduction in risk of recurrence for rectal cancer patients treated with adjuvant 5-FU based regimens (21).
On the other hand, long term results of EORTC 22921 were recently reported (22). This trial employed a 2×2 factorial design to assess the value of adding chemotherapy (5-FU and leucovorin) to preoperative radiotherapy concurrently, post-operatively or in both settings. The addition of chemotherapy, either concurrently with radiotherapy or post-operatively, clearly increased local control rates. However, there was no apparent impact of adjuvant chemotherapy on disease-free or overall survival (22). While these results are in some ways disappointing, it is important to note the very poor rates of adherence to chemotherapy: 82% pre-operatively and just 42.9% post-operatively (5). Both the poor compliance rates and the lack of use of a now standard oxaliplatin-based regimen have caused many to view these negative trial results with skepticism. Regardless, conclusive data is lacking, leaving room for debate as to the optimal incorporation of chemotherapy in rectal cancer.
Multiple investigations have been carried out to improve upon the gains described above, including the incorporation of additional radiosensitizing agents to 5-FU. Though irinotecan, oxaliplatin, bevacizumab, and anti-EGFR therapies have improved survival in the metastatic setting, none have yet proved superior as a radiosensitizer when compared to 5-FU-based chemoradiation (23-25). In addition, apart from oxaliplatin, none of these has conclusively improved outcomes in the adjuvant setting for early stage colorectal cancer (26). The testing of new agents in the adjuvant setting and the development of improved radiosensitizing agents may yet provide gains. However, toxicity appears to be greater with post-operative chemotherapy as well as post-operative chemoradiation, leading to delays in therapy as well as premature discontinuation, undermining its potential benefit. The CAO/ARO/AIO-94 trial demonstrated that post-operative as compared to pre-operative chemoradiation increased rates of grade 3/4 acute (40% vs. 27%) and long term adverse events (24% vs. 14%) (27). Full dose radiation and chemotherapy were administered in just 54% and 50% of post-operatively treated patients as opposed to 92% and 89% of pre-operatively treated patients (27).
Of importance, as highlighted by the results of EORTC 22921, tolerance and compliance with post-operative chemotherapy is consistently dismal, possibly accounting for its inability to demonstrate benefit (5). In fact, greater than one in three patients do not receive post-operative chemotherapy, for a variety of reasons, as recently reported (28). Even in those who ultimately receive chemotherapy, post-operative complications are linked to delays in the initiation of adjuvant chemotherapy and linked to worsened survival (29). Given the lesser toxicity and improved compliance with therapy in the pre-operative setting, there is a growing interest in developing further neoadjuvant treatment strategies for locally advanced rectal cancer. The remainder of this paper will focus on review of recent data and ongoing neoadjuvant therapy efforts. The three major strategies of focus include neoadjuvant chemoradiation followed by chemotherapy, induction chemotherapy followed by chemoradiation, and neoadjuvant chemotherapy alone.
Neoadjuvant chemotherapy alone
As current surgical techniques achieve very good local control rates and the majority of recurrences represent distant metastatic disease, there is a strong argument to be made for turning our focus to improving the delivery of systemic therapy. The current treatment paradigm utilizes nearly 6 weeks of neoadjuvant chemoradiation, 6-8 weeks of recovery prior to surgery, and another 4 weeks of recovery prior to consideration of adjuvant therapy. As such, the standard approach delays the time to initiation of full dose systemic therapy for 4 months, at a minimum. Beginning chemotherapy sooner provides the theoretical advantage of treating micro-metastatic disease earlier, in hope of reducing the incidence of distant recurrence. In addition, as radiotherapy has not improved survival in the vast majority of the studies published, it is possible the added toxicities of this modality may be obviated through use of chemotherapy alone. Radiation related toxicities are not insignificant; there is a substantial incidence of fecal incontinence and sexual dysfunction which tend to be worse with chemoradiation as opposed to radiation alone (30). Patients treated with chemoradiation as compared with surgery alone note worsening of altered bowel habits: more frequent bowel movements per day, more frequent nighttime movements, and a greater incidence of occasional or frequent incontinence necessitating a pad (31).
However, radiotherapy has an established role in this disease. In addition, the MRC CR07/NCIC-CTG C016 comparing pre-operative short course radiotherapy with selective post-operative chemoradiotherapy demonstrated inferior local recurrence rates and DFS with the selective use of chemoradiation, suggesting that we may not be able to pick and choose the patients in whom to administer radiotherapy (32). In subset analysis, the benefit of radiation was maintained in those patients who underwent TME, but TME was not standard in this trial. Also, less than 50% of patients received any chemotherapy. Both of these factors limit the applicability of these results to the current patient population (32,33). Potentially further alleviating this concern, recent updated results of the MERCURY study suggest that pre-operative magnetic resonance imaging (MRI) assessment of the circumferential margin may be very helpful in predicting those patients who will have clear circumferential margins, with a 94% negative predictive value (34). Such assessments may aid in tailoring therapy, limiting the potential harms of withholding any valuable components.
The experience with neoadjuvant chemotherapy as the sole modality is very limited when compared to other approaches. However, initial results are encouraging. A single institutional study of neoadjuvant IFL (weekly irinotecan, 5-FU and leucovorin) was carried out in the early 2000’s in Stage II & III rectal cancers. After 2 months of therapy, 15 of 26 (58%) patients achieved tumor downstaging with one (4%) pathologic complete response (pCR) achieved. A 5-year DFS of 75% was achieved, though there were three pelvic recurrences (35). Importantly, irinotecan is not of proven benefit in adjuvant therapy, and the majority of other efforts focused on oxaliplatin-based therapies. A recent multi-institutional Japanese study evaluated the use of four cycles of neoadjuvant CAPOX (capecitabine + oxaliplatin) and bevacizumab in high risk rectal cancer prior to surgery (T4 in 59.4%, <5 cm from anal verge in 50%). In this 32-patient study, the scheduled chemotherapy was completed by 91% of patients with an R0 resection rate of 90%. pCR was noted in 13% of patients with a total of 37% experiencing good tumor regression (36). A second effort was recently reported from a different group in Japan also utilizing CAPOX and bevacizumab in high risk patients: those with T4 or node positive rectal cancers. Twenty five patients were evaluated, though seven discontinued therapy after 2-3 cycles. One patient (4%) achieved a pCR, and the vast majority were downstaged. Ninety-two percent of patients underwent resection, all with R0 resections. However, post-operative complications were observed in 26% of patients, and at a median follow-up of 31 months, there have been five distant recurrences, including one with accompanying local recurrence (37). While neoadjuvant chemotherapy may be beneficial for high risk rectal cancer, the small numbers and poorer prognosis limit interpretation of the outcomes achieved. There is good reason to proceed with caution in eliminating local therapies for those patients at highest risk of local recurrence.
Average risk patients have also been evaluated through such an approach. A review from Memorial Sloan Kettering of 20 patients with colorectal cancer who were treated initially with FOLFOX +/– bevacizumab demonstrated an impressive pCR rate of 35% (38). Similar results were noted by the same group in a prospective evaluation of rectal cancer patients with standard risk (T3 or N+) tumors >5 cm from the anal verge and without bulky nodes. T4 tumors were not permitted. Thirty two patients were treated with 6 cycles of FOLFOX and bevacizumab followed by TME. Radiation was to be utilized for those without response. In this study, all patients demonstrated tumor regression with a 100% R0 resection rate and a 25% pCR rate. At a mean 53 months follow-up, the local recurrence rate is 0% with a 4-year DFS of 84% (39). While these results are encouraging, the small number of patients significantly hampers our ability to estimate the true benefit of this approach. A summary of select neoadjuvant chemotherapy studies is available in Table 1.
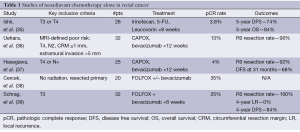
Full table
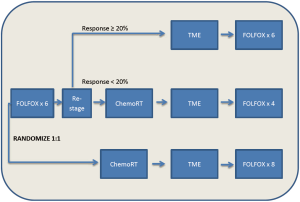
Appropriately, these encouraging results have prompted a prospective randomized trial evaluating this approach: the PROSPECT trial (NCT01515787). The PROSPECT trial is a phase II/III trial from the Alliance for Clinical Trials in Oncology, “The Alliance”, examining the efficacy of 6 cycles of preoperative FOLFOX with the selective use of chemoradiation in patients with non-bulky Stage II/III rectal cancer. Patients are being randomized to pre-operative FOLFOX versus pre-operative chemoradiation, with post-operative treatment left to the discretion of the individual investigator. In the chemotherapy only arm, the use of chemoradiation will be limited to the pre-operative setting in those having less than a 20% reduction in their rectal tumor and the post-operative setting for those patients with positive circumferential margins. MRI will be utilized to guide therapy, with a primary end-point of DFS (Figure 1).
Similar studies evaluating pre-operative chemotherapy are ongoing on overseas. The BACCHUS trial is a medium sized phase II trial evaluating the efficacy and toxicity of 6 cycles of FOLFOX + bevacizumab versus 6 cycles of FOLFOXIRI (5-FU, leucovorin, oxaliplatin, irinotecan), with bevacizumab held in the final cycle for both (NCT01650428). Chemoradiation will only be selectively utilized and the primary outcome is pCR rate. There is also an ongoing 3-arm, randomized phase II trial in China evaluating 4 cycles of pre-operative FOLFOX versus FOLFOX followed by FOLFOX-based chemoradiation versus chemoradiation with 5-FU alone (NCT01211210). The primary end-point is 3-year DFS.
The results from the aforementioned trials will be important in the coming years in shaping the face of rectal cancer therapy, though at present neoadjuvant chemotherapy remains investigational given the limited experience, coupled with the lack of data to predict which locally advanced patients may forgo radiotherapy.
Neoadjuvant chemotherapy followed by chemoradiation
Perhaps the most frequently explored tactic, induction chemotherapy followed by chemoradiation represents an attractive approach. With recognition that distant metastases largely remain the major risk, early systemic therapy is maintained. Still, a positive circumferential margin places patients at greatest risk for local recurrence and a using a combined approach may provide even greater benefit for those patients at elevated risk (distal tumors, >5 mm extramural spread, T4, or bulky nodal disease). As demonstrated in advanced disease, combination chemotherapy with FOLFOX or FOLFIRI induces response in 50-60% of patients with colorectal cancer (40). In sum, induction chemotherapy may allow for early treatment of micrometastatic disease and initial downstaging of the primary tumor. In turn, by following this immediately with chemoradiation, optimal local control may be attained, with the hope of increased complete response rates. It should be noted that this approach, however, has not shown benefit to date in other tumors, such as anal cancer, lung cancer or head and neck cancer. In addition, there is a theoretical risk of selecting for radio-resistant clones by the administration of chemotherapy prior to radiotherapy.
There have been reports on the results of induction chemotherapy followed by chemoradiation in several sizeable trials to date. The EXPERT and GCR-3 studies both examined 12 weeks of induction CAPOX (capecitabine + oxaliplatin) followed by chemoradiation (41,42).The EXPERT trial enrolled 104 patients who were treated with this approach as well as 12 weeks of adjuvant capecitabine. Ninety seven patients underwent resection and 20% of all patients were noted to have a pCR. In this high risk group, 3-year progression free survival (PFS) was 68%, with a 74% 3-year relapse free rate in those patients who underwent resection (41). The Spanish GCR-3 study randomized 108 locally advanced patients to induction CAPOX followed by chemoradiation versus a strategy of chemoradiation followed by post-surgical adjuvant CAPOX. This was also a high risk population. Patients were deemed locally advanced on the basis of MRI; inclusion criteria included involvement of or threated circumferential resection margin (CRM), tumor ≤6 cm from anal verge, resectable cT4 tumors and node positivity. Outcomes between the two arms were comparable, with a pCR rate of 13% vs. 14% (42). Recently with updated follow-up, there is comparable 5-year DFS (60.7% vs. 64.3%) without a significant difference in local relapse (7.1% vs. 1.9%, P=0.36) (43). It is notable that acute grade 3/4 toxicity was observed in 19% of patients who received pre-operative chemotherapy versus 54% of post-operatively treated patients. Not surprisingly, the proportion of patients who completed all 4 cycles of chemotherapy was much improved when administered preoperatively: 94% vs. 57% (42). While not clearly improving outcomes, this supports the notion that a strategy of pre-operative as opposed to post-operative chemotherapy may decrease acute toxicity.
More protracted as well as abridged courses of neoadjuvant therapy have been examined, producing similar results. The CONTRE trial utilized a longer course of 8 cycles of FOLFOX prior to chemoradiation. In a preliminary report, an impressive pCR rate of 33% was demonstrated, albeit in a cohort of just 30 patients (44). Two cycles of CAPOX prior to chemoradiation was evaluated by a Danish Group, producing encouraging results in a phase II study of 85 patients with poor risk rectal cancer. A pCR rate of 25% was obtained, with 5-year for DFS and overall survival (OS) of 63% and 67%, respectively (45). Additionally, a randomized phase II trial utilizing 2 cycles of FOLFOX followed by chemoradiation with chemoradiation alone was also conducted in Belgium. After 57 patients had been enrolled, the trial was closed early for futility based on identical rates of major downstaging (34.5% and 32.1% achieving ypT0-1). Greater grade 3/4 toxicity was seen with induction chemotherapy (46). Finally, utilization of 1 cycle of CAPOX prior to chemoradiation with CAPOX has produced similarly encouraging tumor downstaging rates, pCR rates (23%), and R0 resection rates (98%) (47). Again, it remains difficult to compare merit of the various approaches given substantial issues with patient selection and small numbers.
Additional studies have evaluated the benefit of adding targeted therapies to this treatment paradigm, most notably the EXPERT-C and AVACROSS trials. The EXPERT-C trial compared treatment with four cycles of neoadjuvant CAPOX followed by chemoradiation with or without the addition of cetuximab to the entire pre-operative course. One hundred and sixty five patients with MRI-defined high risk rectal cancer were enrolled. After conception, data emerged supporting cetuximab use only in KRAS wild-type patients. As such, the primary endpoint of complete response was analyzed for the 90 KRAS wild-type patients. Cetuximab increased response rate (95% vs. 73% post-chemoradiation), but complete response rates were similar with or without cetuximab (11% vs. 9%), and there was no difference observed in PFS (48). In a recent follow-up, after a median follow-up of 63.8 months, an exploratory analysis including expanded RAS testing (KRAS non-exon 2 and NRAS) revealed no significant differences in outcomes. However, there was a hint of activity with trends toward improved complete response (15.8% vs. 7.5%, P=0.31), 5-year PFS (78.4% vs. 67.5%, P=0.17) and 5-year OS (83.8% vs. 70%, P=0.20) with cetuximab (49). The AVACROSS trial, demonstrated encouraging results in a poor risk patient population. CAPOX and bevacizumab were used as induction therapy and afterwards radiosensitizers through a multimodality neoadjuvant approach. Though almost all 47 patients (98%) underwent R0 resections and demonstrated a pCR rate of 36%, post-operative complications were abundant. Eleven (24%) patients required repeat surgical interventions (50). Similarly high complication rates have been reported by other groups utilizing neoadjuvant bevacizumab in this manner (24). A summary of select studies utilizing neoadjuvant chemoradiation followed by chemotherapy is available in Table 2.
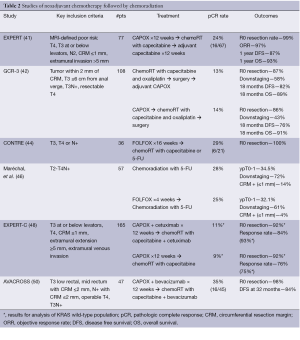
Full table
The verdict is out on whether there is any true improvement in pathologic response rates and more importantly, long term outcomes. As described, the current data comes largely from small phase II studies with great heterogeneity in the proportion of patient with T4 tumors, the dose of radiotherapy administered and timing of surgery. All of these factors may have a substantial impact on pCR rates. The conduct of randomized phase III studies is needed to definitively evaluate this approach. Fortunately, this is an area of active research. The French phase III randomized PRODIGE 23 trial is evaluating a strategy of neoadjuvant FOLFIRINOX prior to chemoradiation versus standard chemoradiation in locally advanced rectal cancer, with plans to enroll 460 patients (NCT01804790). In addition, the ongoing UK COPERNICUS trial is evaluating the feasibility of administering 4 cycles of neoadjuvant FOLFOX prior to short course radiotherapy, followed immediately by surgery (NCT01263171).
Neoadjuvant chemoradiation followed by chemotherapy
A strong argument can be made for the approach of initial chemoradiation followed by chemotherapy, though this has been the least fully explored to this point. Chemoradiation remains the standard neoadjuvant treatment with established benefit. Initial utilization of this modality minimizes risk of interruption due to complications induced by other modalities. As this may be definitive treatment, itself, any detrimental effect that initial chemotherapy may induce is avoided. Moreover, as interest grows in the potential of non-surgical management of rectal cancer, data have suggested that an increased interval between the completion of chemoradiation and surgical evaluation may allow for improved response, namely increased pCR rates, as seen in anal cancer (51). Further validation is needed, and there is potential for worsened fibrosis and more a difficult surgical intervention with prolonged delays between radiotherapy and surgery. Arguing against this approach, the delivery of pelvic radiation may hamper the subsequent ability to deliver full dose chemotherapy, potentially lessening its impact. Further, the response to chemotherapy may not be fully appreciated when chemoradiation is first administered.
Studies of long course chemoradiation followed by pre-operative chemotherapy for locally advanced rectal cancer have been conducted by several groups. Two groups have conducted studies evaluating initial chemoradiation with capecitabine followed by an addition 2-4 weeks of capecitabine prior to surgery. These demonstrated feasibility, without marked increase in acute toxicity or post-operative complications (52,53). At this point, the pCR rates are comparable to other techniques and long term outcome data has not matured. A trial from Italy which used chemoradiation followed by two 3-week cycles of capecitabine (1,250 mg/m2 bid) revealed more encouraging long term follow-up. The pathologic response rate was 18%, with a 5-year DFS of 85.4%. For those patients with tumors ≤6 cm from the anal verge, sphincter preservation rate was 62%. There was a low prevalence of T4 tumors or other high risk features in this study, perhaps accounting for the favorable long term outcomes (54).
As with other approaches, fluoropyrimidine and oxaliplatin based combinations have also been attempted. In a recent study of high risk locally advanced rectal cancer patients, 1 cycle of CAPOX was administered following chemoradiation with CAPOX. pCR was observed in 13 (36.1%) of the 36 patients enrolled (55). An intriguing Dutch report of 50 patients with metastatic, but resectable rectal cancer evaluated a strategy of short course radiotherapy (5×5 Gy) followed by 6 cycles of CAPOX + bevacizumab, which was initiated within 2 weeks of radiotherapy completion. Radical surgical resection was ultimately possible for 72% of all patients treated. The primary rectal tumor was resected in 43 (90%) patients, though a suboptimal R1 resection was achieved in four. In those undergoing primary resection, downstaging was evident in 47% with a pCR rate of 26%. Local recurrence after R0 resection was noted in just 2 (6%) patients (56). Thus, in the metastatic setting, this appears to be a viable approach. At times, short course radiotherapy is not embraced due to the perceived lesser rates of down-staging. The strategy of short course radiotherapy followed immediately by full-dose systemic therapy may allow for optimal downstaging with use of the 5×5 schema, and only minimally delay systemic therapy.
A larger experience has been reported utilizing long-course chemoradiation. In a non-randomized multicenter US study, 144 patients with stage II and III rectal cancer were assigned to one of two study groups. Both received initial 5-FU based chemoradiation. The first group had surgery within 6-8 weeks of completion. The second group was reassessed at 4 weeks and if with evidence of clinical response, patients were treated with two cycles of FOLFOX, followed by surgery 3-5 weeks after completion. Overall pathologic response rates were improved in the group with additional chemotherapy (and delayed surgical intervention), though differences in the pCR rate did not reach significance: 18% vs. 25%, respectively. Importantly, while there was a slight increase in pelvic fibrosis seen, the complication rates were not different between the two groups (51). From this same data set, preliminary results which include a third group of 48 patients have also been reported. In group 3, where two further cycles of FOLFOX were delivered, delaying surgery 4 further weeks, pCR rates increased to 31%, without increased complication rates (57). Thus initial chemoradiation followed by pre-operative chemotherapy appears at least as promising as the other strategies described. A summary of selected studies utilizing this approach is available in Table 3.
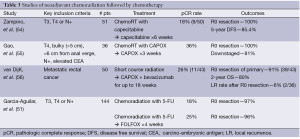
Full table
Multiple trials are ongoing with this approach. The Polish Colorectal Cancer Study Group is conducting a phase III study comparing short-course preoperative radiotherapy followed by three cycles of FOLFOX with conventional chemoradiation to 50.4 Gy with concurrent 5-FU (NCT00833131). The accrual goal is 540 patients and positive results could be practice changing for both radiation and medical oncologists in the United States. An interim analysis revealed no major differences in acute toxicity or local efficacy, with a trend toward improved pCR rates in the short-course radiotherapy group: 21% vs. 9% (58). Equally important is the phase III RAPIDO study which is very similar in design, though goes further in moving the entire current treatment regimen to the pre-operative setting (NCT01558921). Only patients who are deemed high risk by MRI are to be included. In this study, a strategy of short course chemoradiation followed by 6 cycles of CAPOX will be compared with long course chemoradiation. Post-operative adjuvant chemotherapy is left up to the individual investigator.
Conclusions
Outcomes continue to improve in colorectal cancer as affected patients are discovered earlier in the disease process, largely attributable to increased screening efforts. Improved surgical technique, incorporation of pre-operative radiotherapy and the use of adjuvant chemotherapy all appear to confer additional benefit for a large portion of patients. Recent efforts to build upon 5-FU based chemoradiation regimens have yielded negative results. In the meantime, adjuvant colorectal cancer chemotherapy has not progressed further beyond the fluoropyrimidine and oxaliplatin based combination. In rectal cancer, neoadjuvant treatment offers a unique opportunity to improve the current paradigm. There is opportunity to both improve disease free and overall survival outcomes through the differential layering of therapy, as well as to reduce toxicity through the selective use of therapeutic modalities. Selection of the optimal patient population for each paradigm may prove critical in affecting the results and applicability of ongoing studies. Beyond clinical criteria, further biomarker validation may allow for the additional tailoring of therapy moving forward. As always, the support of clinical investigation remains paramount in improving future outcomes for our patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- American Cancer Society: Cancer Facts and Figures 2014. [cited 2014 March 5]; Available online: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/index
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104-17. [PubMed]
- Rutter CM, Johnson EA, Feuer EJ, et al. Secular trends in colon and rectal cancer relative survival. J Natl Cancer Inst 2013;105:1806-13. [PubMed]
- Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [PubMed]
- Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114-23. [PubMed]
- Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005;23:5644-50. [PubMed]
- Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 2006;24:4620-5. [PubMed]
- Havenga K, Enker WE, Norstein J, et al. Improved survival and local control after total mesorectal excision or D3 lymphadenectomy in the treatment of primary rectal cancer: an international analysis of 1411 patients. Eur J Surg Oncol 1999;25:368-74. [PubMed]
- van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011;12:575-82. [PubMed]
- Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827-33. [PubMed]
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006;93:1215-23. [PubMed]
- Khrizman P, Niland JC, ter Veer A, et al. Postoperative adjuvant chemotherapy use in patients with stage II/III rectal cancer treated with neoadjuvant therapy: a national comprehensive cancer network analysis. J Clin Oncol 2013;31:30-8. [PubMed]
- Prolongation of the disease-free interval in surgically treated rectal carcinoma. Gastrointestinal Tumor Study Group. N Engl J Med 1985;312:1465-72. [PubMed]
- Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst 1988;80:21-9. [PubMed]
- Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 1991;324:709-15. [PubMed]
- Wolmark N, Rockette H, Fisher B, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol 1993;11:1879-87. [PubMed]
- O’Connell MJ, Martenson JA, Wieand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med 1994;331:502-7. [PubMed]
- Hofheinz RD, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 2012;13:579-88. [PubMed]
- Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768-74. [PubMed]
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109-16. [PubMed]
- Petersen SH, Harling H, Kirkeby LT, et al. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev 2012;3:CD004078. [PubMed]
- Bosset JF, Calais G, Mineur L, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 2014;15:184-90. [PubMed]
- Horisberger K, Treschl A, Mai S, et al. Cetuximab in combination with capecitabine, irinotecan, and radiotherapy for patients with locally advanced rectal cancer: results of a Phase II MARGIT trial. Int J Radiat Oncol Biol Phys 2009;74:1487-93. [PubMed]
- Dipetrillo T, Pricolo V, Lagares-Garcia J, et al. Neoadjuvant bevacizumab, oxaliplatin, 5-fluorouracil, and radiation for rectal cancer. Int J Radiat Oncol Biol Phys 2012;82:124-9. [PubMed]
- Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol 2011;29:2773-80. [PubMed]
- de Gramont A, Chibaudel B, Bachet JB, et al. From chemotherapy to targeted therapy in adjuvant treatment for stage III colon cancer. Semin Oncol 2011;38:521-32. [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [PubMed]
- Haynes AB, You YN, Hu CY, et al. Postoperative chemotherapy use after neoadjuvant chemoradiotherapy for rectal cancer: Analysis of Surveillance, Epidemiology, and End Results-Medicare data, 1998-2007. Cancer 2014;120:1162-70. [PubMed]
- Tevis SE, Kohlnhofer BM, Stringfield S, et al. Postoperative complications in patients with rectal cancer are associated with delays in chemotherapy that lead to worse disease-free and overall survival. Dis Colon Rectum 2013;56:1339-48. [PubMed]
- Brændengen M, Tveit KM, Bruheim K, et al. Late patient-reported toxicity after preoperative radiotherapy or chemoradiotherapy in nonresectable rectal cancer: results from a randomized Phase III study. Int J Radiat Oncol Biol Phys 2011;81:1017-24. [PubMed]
- Kollmorgen CF, Meagher AP, Wolff BG, et al. The long-term effect of adjuvant postoperative chemoradiotherapy for rectal carcinoma on bowel function. Ann Surg 1994;220:676-82. [PubMed]
- Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet 2009;373:811-20. [PubMed]
- Quirke P, Steele R, Monson J, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 2009;373:821-8. [PubMed]
- Taylor FG, Quirke P, Heald RJ, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol 2014;32:34-43. [PubMed]
- Ishii Y, Hasegawa H, Endo T, et al. Medium-term results of neoadjuvant systemic chemotherapy using irinotecan, 5-fluorouracil, and leucovorin in patients with locally advanced rectal cancer. Eur J Surg Oncol 2010;36:1061-5. [PubMed]
- Uehara K, Hiramatsu K, Maeda A, et al. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: N-SOG 03 Phase II trial. Jpn J Clin Oncol 2013;43:964-71. [PubMed]
- Hasegawa J, Nishimura J, Mizushima T, et al. Neoadjuvant capecitabine and oxaliplatin (XELOX) combined with bevacizumab for high-risk localized rectal cancer. Cancer Chemother Pharmacol 2014;73:1079-87. [PubMed]
- Cercek A, Weiser MR, Goodman KA, et al. Complete pathologic response in the primary of rectal or colon cancer treated with FOLFOX without radiation. J Clin Oncol 2010;28:abstr 3649.
- Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol 2014;32:513-8. [PubMed]
- Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229-37. [PubMed]
- Chau I, Brown G, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol 2006;24:668-74. [PubMed]
- Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol 2010;28:859-65. [PubMed]
- Fernandez-Martos C, Pericay C, Aparicio J, et al. Chemoradiation (CRT) followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant CRT and surgery for locally advanced rectal cancer: Results of the Spanish GCR-3 randomized phase II trial after a median follow-up of 5 years. J Clin Oncol 2014;32:abstr 383.
- Perez K, Pricolo V, Vrees M, et al. A phase II study of complete neoadjuvant therapy in rectal cancer (CONTRE): The Brown University Oncology Group. J Clin Oncol 2013;31:abstr 335.
- Schou JV, Larsen FO, Rasch L, et al. Induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy before total mesorectal excision in patients with locally advanced rectal cancer. Ann Oncol 2012;23:2627-33. [PubMed]
- Maréchal R, Vos B, Polus M, et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol 2012;23:1525-30. [PubMed]
- Koeberle D, Burkhard R, von Moos R, et al. Phase II study of capecitabine and oxaliplatin given prior to and concurrently with preoperative pelvic radiotherapy in patients with locally advanced rectal cancer. Br J Cancer 2008;98:1204-9. [PubMed]
- Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J Clin Oncol 2012;30:1620-7. [PubMed]
- Sclafani F, Gonzalez D, Cunningham D, et al. RAS mutations in EXPERT-C, a randomized phase II trial of neoadjuvant capecitabine and oxaliplatin (CAPOX) and chemoradiotherapy (CRT) with or without cetuximab (C) in MRI-defined, high-risk rectal cancer (RC). J Clin Oncol 2014;32: abstr 489.
- Nogué M, Salud A, Vicente P, et al. Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine-based chemoradiotherapy in magnetic resonance imaging-defined poor-prognosis locally advanced rectal cancer: the AVACROSS study. Oncologist 2011;16:614-20. [PubMed]
- Garcia-Aguilar J, Smith DD, Avila K, et al. Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg 2011;254:97-102. [PubMed]
- Lee KH, Song MS, Park JB, et al. A Phase II Study of Additional Four-Week Chemotherapy With Capecitabine During the Resting Periods After Six-Week Neoadjuvant Chemoradiotherapy in Patients With Locally Advanced Rectal Cancer. Ann Coloproctol 2013;29:192-7. [PubMed]
- Zhu J, Gu W, Lian P, et al. A phase II trial of neoadjuvant IMRT-based chemoradiotherapy followed by one cycle of capecitabine for stage II/III rectal adenocarcinoma. Radiat Oncol 2013;8:130. [PubMed]
- Zampino MG, Magni E, Leonardi MC, et al. Capecitabine initially concomitant to radiotherapy then perioperatively administered in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2009;75:421-7. [PubMed]
- Gao YH, Zhang X, An X, et al. Oxaliplatin and capecitabine concomitant with neoadjuvant radiotherapy and extended to the resting period in high risk locally advanced rectal cancer. Strahlenther Onkol 2014;190:158-64. [PubMed]
- van Dijk TH, Tamas K, Beukema JC, et al. Evaluation of short-course radiotherapy followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin and subsequent radical surgical treatment in primary stage IV rectal cancer. Ann Oncol 2013;24:1762-9. [PubMed]
- J. Garcia-Aguilar, J. Marcet, T. Coutsoftides, et al. Impact of neoadjuvant chemotherapy following chemoradiation on tumor response, adverse events, and surgical complications in patients with advanced rectal cancer treated with TME. J Clin Oncol 2011;29:abstr 3514.
- Bujko K, Nasierowska-Guttmejer A, Wyrwicz L, et al. Neoadjuvant treatment for unresectable rectal cancer: an interim analysis of a multicentre randomized study. Radiother Oncol 2013;107:171-7. [PubMed]


