A case of necrotizing vasculitis with panniculitis, during sorafenib treatment for hepatocellular carcinoma, appeared in disease progression
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the third most common cause of death from cancer (1).
When diagnosed in early stages, HCC is amenable to potentially curative treatments such as surgery (resection or liver transplantation), or loco-regional procedures like radiofrequency ablation (2). Unfortunately, a dismal prognosis is reserved for HCC when an advanced stage has been diagnosed or after progression to loco-regional therapies, in part due to the lack of effective treatment options and in part due to the underlying liver disease (2-4). Before sorafenib, no systemic therapy has improved survival in patients with advanced HCC (5).
Sorafenib (Nexavar, Bayer AG) is a small molecule that inhibits the serine-threonine kinases Raf-1 and B-Raf and the tyrosine kinase activity of vascular endothelial growth factor receptors (VEGFRs) 1, 2, and 3 and platelet-derived growth factor receptor β (PDGFR-β) (6,7).
Sorafenib inhibits tumour-cell proliferation, tumour angiogenesis and increases the rate of apoptosis in a wide range of tumour models (6,7). Sorafenib is approved for advanced renal carcinoma treatment (8) and for advanced HCC (9).
In advanced HCC, sorafenib was superior, when compared to placebo, in terms of overall survival (10.7 vs 7.9 months, hazard ratio 0.69; 95% confidence interval, 0.55 to 0.87; P<0.001) (9).
Like all tyrosine kinase inhibitors (TKI), sorafenib has been associated with several dermatologic toxicities (10). Development of skin toxic effects with EGFR inhibitors has been related with a better treatment response (11).
Case report
We report a case of panniculitis and necrotizing vasculitis due to sorafenib, appeared in disease progression in an 80-year-old white man affected by HCC, HCV-related.
The patient had a clinical history of arterial hypertension, idiopathic myelofibrosis in treatment with oncocarbide from 2008 and chronic obstructive pulmonary disease (COPD); HCC was diagnosed in December 2012 and treated with radiofrequency ablation in January 2013.
In October 2013, a CT scan showed local progression with multiple hepatic lesions and an alpha fetoprotein (AFP) value of 1,362 ng/mL.
In December 2013, sorafenib 400 mg twice daily was started; after 7 days, due to asthenia G3 and significant abdominal pain, treatment was discontinued. After the regression of symptoms, sorafenib was restarted with daily dose reduction to 600 mg, with good tolerance.
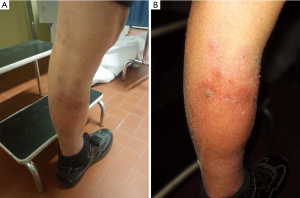
In February 2014, after three months of therapy, patient complained left leg pain and edema. Clinical examination showed several harsh and painful skin lesions on palpation (Figure 1A,B); AFP was 3,179 ng/mL, doubled compared to baseline.
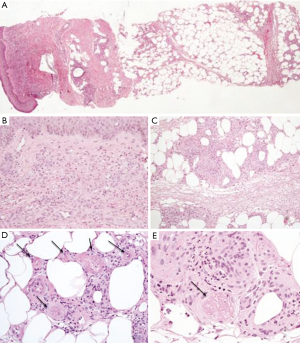
A skin biopsy revealed a perivascular lympho-granulocytic inflammatory infiltrate involving dermal and subcutaneous tissue. Epidermis was slightly hyperplastic. Inflammatory infiltrate was heavier in subcutaneous fat than in superficial dermis and it was associated with lobular panniculitis and necrotizing vasculitis. At this level, the blood vessels showed fibrinoid necrosis and thrombosis. The periodic acid Schiff (PAS) staining method, performed to detect fungal infection, resulted negative. The morphological findings were consistent with a panniculitis associated with necrotizing vasculitis (Figure 2).
Sorafenib was interrupted with the resolution of cutaneous lesions in 2 weeks. A CT scan confirmed the progression of liver lesions.
Discussion
Cutaneous reactions are frequent in patients treated with TKI and some cases of vasculitis have been described in literature. Maculopapular rash, papulopustular rash, bullous dermatitis, vasculitis, drug rash with eosinophilia and systemic symptoms (DRESS) or panniculitis have been observed during sorafenib treatment (10). Three different hypotheses have been generated to explain the development of cutaneous toxicity. The first hypothesis concerns the inhibition of mitogen-activated protein kinase, stress-activated protein kinase, and VEGF pathways. This leads to keratinocyte proliferation and focal apoptosis carrying to keratosis pilaris, epidermal inclusion cysts and keratoacanthomas. The second hypothesis is that of the inhibition of c-kit or RAF kinase resulting in keratinocyte injury. Histopathologically, cutaneous lesion is seen as a focal epithelial damage with dyskeratotic keratinocytes and reactive epithelial changes in the basal layer of the epidermis and in eccrine sweat ducts. The last and third hypothesis is that of apoptosis induction in endothelial-cells due to direct anti-VEGFR or anti-PDGFR effects on dermal endothelial cells (12).
Vascular lesions have been also described in patients with HCC, but they are more frequent in HBV-related HCC (13) and seem related to an autoimmune mechanism.
Other cases of vascular lesions have been described during treatment with sorafenib (13) but only a few have been documented histologically.
In our case, skin lesions were located only on the left leg, without other cutaneous lesions; biopsy described lobular panniculitis and necrotizing vasculitis.
Our case, like other clinical cases described in literature, shows the extreme variability of skin lesions that may occur during treatment with sorafenib and other TKIs.
Very interesting the lack of correlation between response and toxicity; diversely than other cases reported with other TKI, we found, indeed at the same time, progression disease and skin toxicity. Liver and renal function was normal so there was no reason for suspecting a drug accumulation.
To our knowledge, this is the first case of localized vasculitis histologically confirmed, due to a TKI and appeared during progression disease. It seems that vascular lesions can appear during every sorafenib treatment phase regardless of the effectiveness and that toxicity is rapidly reversible with TKI suspension.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-17. [PubMed]
- Bruix J, Sherman M, Practice Guidelines Committee, et al. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36. [PubMed]
- Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421-30. [PubMed]
- Lopez PM, Villanueva A, Llovet JM. Systematic review: evidence-based management of hepatocellular carcinoma--an updated analysis of randomized controlled trials. Aliment Pharmacol Ther 2006;23:1535-47. [PubMed]
- Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099-109. [PubMed]
- Chang YS, Adnane J, Trail PA, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol 2007;59:561-74. [PubMed]
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125-34. [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [PubMed]
- Faye E, Bondon-Guitton E, Olivier-Abbal P, et al. Spontaneous reporting of serious cutaneous reactions with protein kinase inhibitors. Eur J Clin Pharmacol 2013;69:1819-26. [PubMed]
- Strumberg D, Awada A, Hirte H, et al. Pooled safety analysis of BAY 43-9006 (sorafenib) monotherapy in patients with advanced solid tumours: Is rash associated with treatment outcome? Eur J Cancer 2006;42:548-56. [PubMed]
- Pragasam V, Verma R, Vasudevan B. Sorafenib and sunitinib: A dermatologist’s perspective. Indian Dermatol Online J 2014;5:1-3. [PubMed]
- Glinkov S, Krasnaliev I, Atanassova M, et al. Hepatocellular carcinoma associated with paraneoplastic erythema nodosum and polyarthritis. J Hepatol 2003;39:656-7. [PubMed]