A patient with cholangiocarcinoma demonstrating pathologic complete response to chemotherapy: exploring the role of neoadjuvant therapy in biliary tract cancer
Introduction
Cholangiocarcinoma (CCA), which is traditionally thought to arise from the cholangiocytes of the epithelial bile ducts anywhere along the intra- or extra-hepatic biliary tree, remains a relatively rare cancer, comprising approximately 3% of all gastrointestinal malignancies (1,2). However, the incidence of CCA appears to be on the rise in Western populations, particularly intrahepatic CCA, where mortality also appears to be increasing (3-5). Early stage CCA can be cured in some instances with surgical resection, but the majority of patients are diagnosed when the cancer is unresectable due to local extent of the tumor (i.e., involvement of adjacent blood vessels or extension into both hepatic lobes beyond the secondary radicals), or from metastatic dissemination (6). A number of patients may also present with marginally resectable tumors; however, incomplete resections (R1/R2), even with the addition of postoperative chemotherapy and/or radiation, result in outcomes comparable to those of individuals not undergoing surgical resection (7,8). Such patients may be candidates for neoadjuvant therapy to increase the likelihood of R0 resection, but this strategy of delivering preoperative treatment for either localized or locally advanced CCA has not been studied extensively and does not currently represent an accepted standard of care. We present here the case of a patient with localized extrahepatic CCA in whom surgical resection was delayed secondary to a concurrent diagnosis of non-small cell lung cancer (NSCLC), who ultimately achieved a complete pathologic response of the CCA from gemcitabine-based chemotherapy alone.
Case
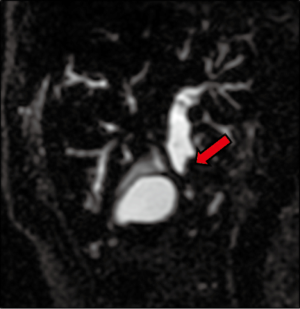
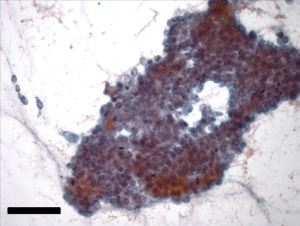
A 64-year-old gentleman, previously healthy, presented with painless jaundice, fatigue, pruritis, fatty food intolerance, and a 7 kg weight loss over a 2-month period. Laboratory studies revealed abnormal liver function tests, including an elevated total bilirubin level of 9.5 mg/dL. Diagnostic imaging consisted of abdominal ultrasound followed by magnetic resonance cholangiopancreaticogram, demonstrating a 2 cm lesion in the common bile duct (CBD) with associated intrahepatic and proximal extrahepatic biliary ductal dilatation as well as borderline enlarged porta hepatis lymph nodes (Figure 1). Endoscopic ultrasound was then performed, notable for moderate diffuse intrahepatic ductal dilation with biliary dilation extending to the mid extrahepatic CBD, where abrupt narrowing was seen secondary to an ill-defined, relatively homogeneous, 10.3 mm area suggestive of malignant stricture. The hepatic artery, portal vein, splenoportal confluence, and superior mesenteric vessels were all distinct and intact, and there were no focal pancreatic lesions or enlarged periceliac lymph nodes. Fine needle aspiration of the mid-CBD stricture demonstrated adenocarcinoma (Figure 2), with immunohistochemical (IHC) stains positive for cytokeratin (CK) 7, deleted in pancreatic cancer 4 (DPC4), and (focally) CK20, consistent with an extrahepatic CCA.
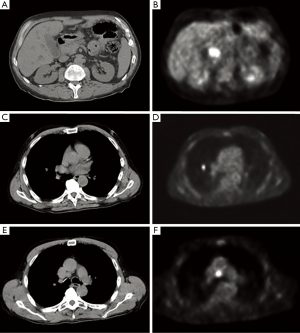
Further staging was performed to determine whether the patient was an appropriate operative candidate. CT-PET scan confirmed a focal area of hypermetabolism corresponding to the site of his primary tumor, measuring approximately 2.9 cm × 1.9 cm × 2.7 cm with a standardized uptake value (SUV) of 9.5. However, in addition, a 1.3 cm × 1.1 cm hypermetabolic nodule in the right middle lobe of the lung (SUV 6.4) and an enlarged 1.9 cm × 1.4 cm precarinal lymph node (SUV 8.1) were also appreciated (Figure 3).
Further diagnostic evaluation included an endobronchial ultrasound-guided fine needle aspiration of the precarinal lymph node, which yielded non-diagnostic material; and an endoscopic retrograde cholangiopancreaticogram, which confirmed a long irregular stricture with lobulated tumor within the proximal to mid extrahepatic duct. A 7 French double-pigtail endobiliary stent was placed through this strictured area resulting in successful biliary decompression. To definitively address the patient’s suspicious pulmonary findings, he was taken for en bloc right middle pulmonary lobectomy and radical superior mediastinal and hilar lymph node dissection. Pathology showed a 1.5 cm intermediate-grade adenocarcinoma with a mixed lepidic and acinar growth pattern, staining positive for napsin A, thyroid transcription factor (TTF)-1, CK7, and CK20, by IHC. This pattern was clearly distinct from his primary bile duct tumor and supported a second separate diagnosis of primary pulmonary adenocarcinoma, AJCC stage IIIA (pT1aN2) based on involvement of 4 of 17 positive lymph nodes.
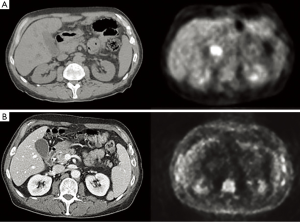
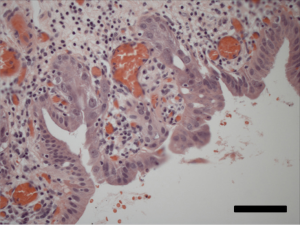
Following an uneventful postoperative recovery, the patient underwent repeat CT-PET confirming no locoregional or distant progression of his CCA during the intervening 3-month period. To avoid two successive operations within such a short timeframe, the clinical team opted to initiate chemotherapy with the combination of gemcitabine and cisplatin, which was felt to be appropriate both as adjuvant therapy for his stage III NSCLC and as neoadjuvant therapy for his CCA. The patient received three cycles of gemcitabine at a starting dose of 1,000 mg/m2 plus cisplatin 25 mg/m2, both drugs administered on days 1 and 8 of a 21-day cycle, with treatment complicated by recurrent asymptomatic neutropenia necessitating gemcitabine dose reduction and growth factor support after cycle 1. Repeat PET/CT imaging thereafter noted minimal metabolic activity at the level of the CBD (SUV 2.8) (Figure 4); and no suggestion of early relapse of his NSCLC. He was then taken for pylorus-sparing pancreaticoduodenectomy (Whipple procedure), 5.5 months from the date of his prior thoracic surgery and 7.5 months from his original CCA diagnosis. Intraoperative frozen section analysis of the proximal and distal bile duct margins was performed to ensure there was no high-grade dysplasia or invasive cancer. Final surgical pathology demonstrated no residual in situ or invasive carcinoma, with only focal mucosal denudation, areas of acute and chronic inflammation, and biliary intraepithelial neoplasia I (BilIN I) at the distal CBD margin (Figure 5). Zero of 17 lymph nodes were involved with tumor.
The patient recovered well from this second operation and proceeded to undergo thoracic irradiation, as he was felt to be at highest risk for relapse of his NSCLC, without further chemotherapy. He has since remained without evidence of recurrence of either his CCA or his NSCLC, now 1.5 years from the time of his original diagnoses.
Discussion
CCA is a morbid disease, with overall 1- and 5-year survival rates of 25% and <5%, respectively (9). Surgical resection, which may require partial hepatectomy and/or Whipple procedure in addition to biliary reconstruction depending on the location and the proximal and distal extent of the tumor, represents the only potentially curative option. However, post-resection 5-year recurrence rates range from 60-90% (9), with most cases recurring locally (10), highlighting the importance of exploring the role for adjuvant and/or neoadjuvant therapy in an effort to reduce relapse risk.
While adjuvant therapy is often used following resection of either intra- or extra-hepatic CCA, this approach is not well supported by clinical evidence to date. Only two randomized phase III trials have addressed the role of adjuvant chemotherapy in combined populations of CCA, gallbladder cancer, and other tumor types. Takada et al. performed a randomized trial of postoperative adjuvant therapy with mitomycin C (MMC) and 5-fluorouracil (5-FU) compared to surgery alone in patients with resectable pancreatic, gallbladder, bile duct, and ampulla of Vater carcinomas. Specific to the bile duct subgroup (n=118), adjuvant chemotherapy conferred no benefit in terms of 5-year overall or disease-free survival (11). A subsequent trial performed by the European Study Group for Pancreatic Cancer (ESPAC-3) compared chemotherapy (either 5-FU/folic acid or gemcitabine) to observation alone in patients with resected periampullary carcinomas (12). In the subgroup of patients with CCA (n=96), survival benefit was not demonstrated from administration of chemotherapy (median survival times for observation, gemcitabine, and 5-FU of 27.2, 19.5, and 18.3 months, respectively). Additional data are expected from a multicenter randomized phase III BILCAP study (NCT003635840) in which patients with gallbladder or bile duct carcinomas are randomized after surgery to receive adjuvant capecitabine vs. observation.
A recent meta-analysis conducted by Horgan et al. inclusive of 6,712 patients with biliary tract cancers did find a nonsignificant improvement in overall survival with use of adjuvant therapy (chemotherapy and/or radiation) following surgery, compared with surgery alone [pooled odds ratio (OR), 0.74; P=0.060]. The survival benefit was greater, reaching statistical significance, in the subset of patients with lymph node-positive disease (OR, 0.49; P=0.004) and those undergoing R1 resections (OR, 0.36; P=0.002) (13). On the above bases, the National Comprehensive Cancer Network (NCCN) currently recommends adjuvant chemotherapy with a fluoropyrimidine- or gemcitabine-based regimen in the setting of resected CCA (14). Alternatively, fluoropyrimidine-based chemoradiation can be offered, although given the lack of sufficient randomized controlled trials, the relative contributions of chemotherapy and radiation are not clearly defined. In a recent Surveillance, Epidemiology, and End Results (SEER) analysis of 1,491 patients with resected extrahepatic CCA, use of adjuvant radiation was in fact not associated improved long-term survival (15).
There is even less evidence in support of neoadjuvant therapy for CCA. Specifically, no randomized trials addressing this issue have been conducted, and most data are derived from retrospective single-institution reports or non-randomized studies (16-21). The benefits of neoadjuvant therapy must be weighed against the potential harm of delaying surgery, especially when CCA is resectable at presentation. In a retrospective review including 94 resectable CCA patients, investigators from MD Anderson Cancer Center reported that neoadjuvant therapy delayed surgery by an average of 6.8 months and resulted in decreased survival relative to immediate resection, from 53.5 to 42.3 months (16).
While data do not support the use of neoadjuvant therapy for clearly resectable CCA, this approach is conceptually appealing when the goal is to downstage patients with borderline resectable disease to increase the likelihood of R0 resection. McMasters et al. first reported treating nine patients (six with unresectable disease) with 5-FU and external beam radiotherapy, after which all patients underwent successful R0 resection (17). Nelson and colleagues subsequently reported their experience treating 12 patients (10 unresectable) with a similar regimen, with or without the addition of brachytherapy, and achieved a 91% R0 resection rate (19). Most recently, Kato et al. achieved an 18% R0 rate in 22 patients with locally advanced disease receiving preoperative gemcitabine without radiation (20). Other treatment modalities, such as yttrium-90 radioembolization (22) for advanced intrahepatic disease or photodynamic therapy (8,18) for hilar disease, represent intriguing approaches but remain inadequately studied. In a recent systematic review of hilar CCA, Grendar et al. concluded there is weak (Level 4) evidence that neoadjuvant treatment improves resectability and survival for patients with unresectable disease (7). Current NCCN guidelines do not recommend the use of neoadjuvant therapy for any stage or location of CCA.
The most promising application of neoadjuvant therapy is in select patients with locally advanced hilar CCA who are unresectable due to underlying liver disease or tumor stage, but without lymph node involvement or intrahepatic or extrahepatic metastases. In the 1990’s, investigators at the Mayo Clinic and the University of Nebraska developed protocols to treat such patients with fluoropyrimidine-based chemoradiation followed by orthotopic liver transplant. Of note, a significant portion of these carefully selected cohorts developed nodal metastases or disease progression which precluded transplant, but in those that completed the protocol, long-term disease-free survival rates of 45% (23) and 92% (24) were achieved. These results have been corroborated in larger single-center retrospective reports (25-28) with, at one center, long-term recurrence and survival rates favorably comparable to those who underwent complete conventional resection (29). In the only multicenter analysis to date, Darwish Murad and colleagues pooled the experiences of 12 transplant centers that had, from 1993-2010, collectively treated 287 patients under similar protocols (30). Five-year recurrence free survival in an intent-to-treat analysis was 53%, while post-transplant 5-year survival was 65%. This study also highlighted the necessity for careful patient selection; those transplanted outside of the United Network for Organ Sharing criteria for prioritization (i.e., those with tumor mass >3 cm, metastatic disease, history of direct tumor biopsy, or previous malignancy within five years) had significantly worse recurrence-free survival (HR, 2.98; 95% CI, 1.79-4.95). Though of limited generalizability, these data are the most convincing evidence in support of neoadjuvant therapy for CCA.
In the case reported here, the diagnoses of two synchronous tumors presented challenging treatment decisions. Our patient would ordinarily have been an appropriate candidate for up-front surgical resection of his extrahepatic CCA in the absence of an established role for neoadjuvant therapy in the context of clearly localized disease. However, his concurrent diagnosis of node-positive NSCLC required more immediate attention and led to the delay of bile duct surgery. Following his thoracic operation, the decision was made to deliver chemotherapy for 3 months to (I) allow more postoperative recovery time prior to a second planned major cancer operation; (II) treat his NSCLC in the adjuvant setting; (III) prevent the progression of his CCA; and (IV) ensure favorable biology of both tumors to avoid major abdominal surgery if it were unlikely to be curative. A gemcitabine/cisplatin regimen was selected because it is both the standard front line regimen for advanced/metastatic CCA, as established by two multicenter randomized phase III trials in the UK and Japan (31,32), and also represents a reasonable option for NSCLC (33).
Most striking in this case was the achievement of a pathologic complete response. Previous studies of chemotherapy alone are largely limited to patients with advanced or metastatic CCA in whom surgery is rarely performed; thus it is impossible to determine the frequency of pathologic responses in this context. However, only two complete radiologic responses were reported in a combined total of 494 patients in the two largest randomized trials, so it can be assumed that pathologic complete responses to chemotherapy are either nonexistent or exceptionally rare. Even with combined-modality therapeutic approaches that incorporate radiation, complete responses occur very infrequently (17,19,27-29), and some of the reported cases may represent misdiagnoses due to use of radiographic criteria or cytology brushings for initial disease confirmation (34). In fact, only one case of pathologic complete response to chemotherapy has been previously reported, in a patient with metastatic intrahepatic CCA receiving a salvage regimen consisting of cisplatin, 5-FU, doxorubicin, and interferon K (35). This regimen is not currently recommended due to its limited clinical benefit and excessive toxicity (36), and thus ours is the first documented case of CCA achieving a pathologic complete response to a more conventional chemotherapeutic regimen without the addition of concurrent or sequential radiation.
In conclusion, we propose that strategies evaluating neoadjuvant therapy for locally advanced or even localized CCA should be formally evaluated in larger multicenter studies. Such studies should include both R0 resection rate and pathologic complete responses as endpoints in addition to survival outcomes. Whether pathologic complete response correlates to improved survival in CCA, as is the case with other gastrointestinal malignancies in which neoadjuvant therapy is given (37,38), remains to be seen. Our case also suggests that select patients may demonstrate robust or even pathologic complete responses to chemotherapy alone, and thus a comparison of this therapeutic strategy to combined-modality therapy (chemotherapy plus radiation) may be warranted in prospective trial design.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Sia D, Tovar V, Moeini A, et al. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene 2013;32:4861-70. [PubMed]
- Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis 1994;14:109-14. [PubMed]
- Faris JE, Zhu AX. Targeted therapy for biliary tract cancers. J Hepato Biliary Pancreat Sci 2012;19:326-36. [PubMed]
- Khan SA, Thomas HC, Davidson BR, et al. Cholangiocarcinoma. Lancet 2005;366:1303-14. [PubMed]
- Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001;33:1353-7. [PubMed]
- Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology 2008;48:308-21. [PubMed]
- Grendar J, Grendarova P, Sinha R, et al. Neoadjuvant therapy for downstaging of locally advanced hilar cholangiocarcinoma: a systematic review. HPB 2014;16:297-303. [PubMed]
- Witzigmann H, Berr F, Ringel U, et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann Surg 2006;244:230-9. [PubMed]
- Mosconi S, Beretta GD, Labianca R, et al. Cholangiocarcinoma. Crit Rev Oncol Hematol 2009;69:259-70. [PubMed]
- Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma. Cancer 2003;98:1689-700. [PubMed]
- Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? Cancer 2002;95:1685-95. [PubMed]
- Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: The espac-3 periampullary cancer randomized trial. JAMA 2012;308:147-56. [PubMed]
- Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934-40. [PubMed]
- National Comprehensive Cancer Network. Hepatobiliary Cancers NCCN Guidelines (Version 2.2014). Available online: http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf, accessed June 13, 2014.
- Vern-Gross TZ, Shivnani AT, Chen K, et al. Survival outcomes in resected extrahepatic cholangiocarcinoma: effect of adjuvant radiotherapy in a surveillance, epidemiology, and end results analysis. Int J Radiat Oncol Biol Phys 2011;81:189-98. [PubMed]
- Glazer ES, Liu P, Abdalla EK, et al. Neither neoadjuvant nor adjuvant therapy increases survival after biliary tract cancer resection with wide negative margins. J Gastrointest Surg 2012;16:1666-71. [PubMed]
- McMasters KM, Tuttle TM, Leach SD, et al. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am J Surg 1997;174:605-8. [PubMed]
- Wiedmann M, Caca K, Berr F, et al. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma: a phase II pilot study. Cancer 2003;97:2783-90. [PubMed]
- Nelson JW, Ghafoori AP, Willett CG, et al. Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys 2009;73:148-53. [PubMed]
- Kato A, Shimizu H, Ohtsuka M, et al. Surgical resection after downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer: a retrospective single-center study. Ann Surg Oncol 2013;20:318-24. [PubMed]
- Katayose Y, Rikiyama T, Motoi F, et al. Phase I trial of neoadjuvant chemoradiation with gemcitabine and surgical resection for cholangiocarcinoma patients (NACRAC study). Hepatogastroenterology 2011;58:1866-72. [PubMed]
- Mouli S, Memon K, Baker T, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol 2013;24:1227-34. [PubMed]
- Sudan D, DeRoover A, Chinnakotla S, et al. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant 2002;2:774-9. [PubMed]
- De Vreede I, Steers JL, Burch PA, et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl 2000;6:309-16. [PubMed]
- Rosen CB, Heimbach JK, Gores GJ. Surgery for cholangiocarcinoma: the role of liver transplantation. HPB 2008;10:186-9. [PubMed]
- Duignan S, Maguire D, Ravichand CS, et al. Neoadjuvant chemoradiotherapy followed by liver transplantation for unresectable cholangiocarcinoma: a single-centre national experience. HPB 2014;16:91-8. [PubMed]
- Panjala C, Nguyen JH, Al-Hajjaj AN, et al. Impact of neoadjuvant chemoradiation on the tumor burden before liver transplantation for unresectable cholangiocarcinoma. Liver Transpl 2012;18:594-601. [PubMed]
- Welling TH, Feng M, Wan S, et al. Neoadjuvant stereotactic body radiation therapy, capecitabine, and liver transplantation for unresectable hilar cholangiocarcinoma. Liver Transpl 2014;20:81-8. [PubMed]
- Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg 2005;242:451-8; discussion 458-61. [PubMed]
- Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012;143:88-98. [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [PubMed]
- Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 2010;103:469-74. [PubMed]
- Cardenal F, López-Cabrerizo MP, Antón A, et al. Randomized phase III study of gemcitabine-cisplatin versus etoposide-cisplatin in the treatment of locally advanced or metastatic non–small-cell lung cancer. J Clin Oncol 1999;17:12-8. [PubMed]
- Kelley RK, Hirose R, Venook AP. Can we cure cholangiocarcinoma with neoadjuvant chemoradiation and liver transplantation? Time for a multicenter trial. Liver Transpl 2012;18:509-13. [PubMed]
- Slupski MW, Szczylik C, Jasinski MK. Unexpected response to systemic chemotherapy in case of primarily nonresectable advanced disseminated intrahepatic cholangiocarcinoma. World J Surg Oncol 2007;5:36. [PubMed]
- Patt YZ, Hassan MM, Lozano RD, et al. Phase II trial of cisplatin, interferon α-2b, doxorubicin, and 5-fluorouracil for biliary tract cancer. Clin Cancer Res 2001;7:3375-80. [PubMed]
- Meredith KL, Weber JM, Turaga KK, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol 2010;17:1159-67. [PubMed]
- Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835-44. [PubMed]


