Intestinal metaplasia and anastomotic recurrence of gastric carcinoma
Introduction
Gastric adenocarcinoma is a tumour derived from the lining mucosa which usually presents late in its natural history. This advanced cancer is the sixth most common and causes approximately 7,600 deaths per annum in the UK although the incidence has inexplicably been falling for several decades (1,2). The most widely accepted histological classification of gastric carcinoma is that by Lauren who divided the tumours into two main types. Those which formed glandular structures were known as intestinal (53%) whereas those without any structure and secreting mucin were known as diffuse type carcinomas (33%). The remaining 14% had a mixed appearance with elements from both types and were regarded as unclassified (3). The number of patients who undergo endoscopic surveillance after subtotal gastrectomy has recently been increasing, which is due to the improved survival rate in gastric cancer patients (4,5). However, it is difficult to detect early gastric cancer (EGC) during endoscopic surveillance because the remnant stomach is usually deformed after surgical resection, and the mucosal changes at the gastric stump are severe due to bile reflux (6). The incomplete type intestinal metaplasia (IM) (type III) has been shown to increase the relative risk of gastric cancer by a factor of 4.58 (7,8). Fortunately, it is less frequent than the complete type (21.5% of IM) (9). Therefore, it would be very helpful if the endoscopic features of EGC or IM that exist on a specific location of the gastric remnant such as at the anastomosis site or at a non-anastomosis site, could be characterized (4-7).
Method
Electronic searches of the medline (PubMed) database, Cochrane library, and science citation index was performed to identify original published studies on IM, gastric carcinoma and anastomotic recurrence.
Epidemiology
The incidence of gastric cancer is high in Eastern Asia, Eastern Europe, and Andean-Latin America (10,11). Epidemiologically, the classification of gastric carcinoma has proved most useful as the two types may represent different diseases and as a result has different aetiological factors. The intestinal type which is thought to arise from IM in the stomach is more prevalent in the older age group contrasting with the diffuse type which has an equal sex incidence and occurs at a younger age. The intestinal type is more common in areas of high incidence, e.g., Japan and the Far East, whereas the diffuse type occurs equally irrespective of incidence rates. Furthermore, the excess incidence of intestinal type is associated with the high mortality seen in areas of high incidence. Conversely, the reduction in mortality in areas of decreasing incidence is associated with a reduction of incidence of the intestinal type. Furthermore, this reduction is associated with the decrease in incidence in the distal stomach suggesting that the intestinal type is a disease of the gastric antrum. This suggests that it is certain dietary or environmental factors which are important in the development of the intestinal lesion, whereas the diffuse lesion which is what we get left with, with the decrease incidence of the disease is often related to hereditary factors (12,13). In the United States, several ethnic populations have a high cancer risk, including African-Americans, native Americans, and immigrants from high-risk regions (14). Endoscopic surveillance has been proposed and advocated for populations at risk (15). Because the much lower incidence of gastric cancer in the United States and other Western countries does not justify screening, it thus presents late in its natural history in these countries (16).
Risk factors and natural history
Risk factors for IM include Helicobacter pylori (H. pylori)infection, high salt intake, smoking, alcohol consumption, and chronic bile reflux (17). The development of gastric adenocarcinoma of the intestinal type is thought to progress sequentially through four stages: non-atrophic gastritis, multifocal atrophic gastritis, IM, and dysplasia. Chronic H. pylori infection induces chronic inflammation in the gastric mucosa, which may progress to atrophy and IM which is a precursor to gastric adenocarcinoma (18,19). IM initially appears at the antrum-corpus junction, especially at the gastric angularis. As atrophy and metaplastic changes advance, they tend to extend to the antrum and corpus, and dysplastic foci may eventually appear.
Correa have proposed that there is a progression from normal gastric mucosa to carcinoma in high risk populations (20). The initial change is early onset superficial gastritis which, although reversible, is triggered by a variety of agents. It may progress to chronic gastritis which may be associated with varying degrees of atrophy. Within the areas of gastric atrophy IM may occur and particularly in those areas where the metaplasia is similar to large bowel epithelium (type III IM), dysplasia may supervene and hence carcinoma of the intestinal type. In high risk areas both IM and chronic gastritis are found in association with intestinal type cancer. Patients with chronic atrophic gastritis (CAG) associated with autoimmune pernicious anaemia are at risk from gastric cancer and the gastric mucosa from the early stage of the disease has similar features of intestinal metaplasia. IM in patients with the diffuse type of cancer is no different from the general population (19).
Pathology of IM
Gastric IM is categorized histopathologically into incomplete and complete types. Incomplete IM resembles colonic epithelium with multiple, irregular mucin droplets of variable size in the cytoplasm and absence of a brush border. Complete IM resembles small intestinal epithelium with eosinophilic enterocytes, a brush border, goblet cells, and variable Paneth cells (Figure 1). H. pylori colonization can be patchy in complete IM type (21-23).
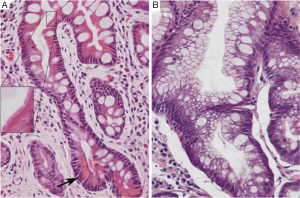
Patients with incomplete IM should undergo endoscopic gastric mapping to define the extent of IM and to rule out dysplasia or adenocarcinoma. Gastric mapping involves biopsies of the six zones of the stomach (antrum greater curve, antrum lesser curve, gastric angularis, body greater curve, body lesser curve, and fundus) and any visible lesions. Biopsies from each zone should be collected into a separate specimen bottle. Complete IM is associated with a lower risk of gastric cancer. Therefore, in the absence of other risk factors for gastric cancer, patients with complete IM do not need long-term endoscopic surveillance (17).
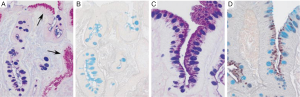
The changes of IM in the stomach can be classified according to the type of mucins. It is really the type 2-b that is important in the subsequent development of malignancy, and one needs to get sulphomucin and sialomucin staining to establish that it is appropriate intestinal metaplasia that requires subsequent follow up (Figure 2) (24-26). Elevated serum pepsinogen level has been proposed as a marker of extensive gastric atrophy (27). Currently, there are no reliable markers of gastric dysplasia or cancer. Patients with high-grade dysplasia (HGD) or carcinoma in situ confirmed by at least two gastrointestinal pathologists should undergo surgical or endoscopic resection because of the high probability of coexisting or metachronous invasive carcinoma (28). IM of the cardia and Barrett’s esophagus differ in their risk for malignant transformation (17).
There are no widely accepted guidelines on the management of gastric IM. Recently, the European Society of Gastrointestinal Endoscopy and other European academic societies have developed evidence-based guidelines on the management of patients with gastric IM. The recommendations emphasize the increased cancer risk in patients with gastric atrophy and IM and the need for adequate staging in the case of HGD (6).
Pathological features of residual, recurrent and newly developed cancer of the remnant stomach
The definition of remnant gastric cancer (RGC) has changed since the first description of gastric cancer after partial gastrectomy for benign peptic ulcer disease in the early 1920s (28,29). Recent definition of RGC is any gastric cancer that develops on the remnant gastric mucosa after a partial gastrectomy because of gastric cancer or benign gastric disease. Kaminishi et al. have classified RGCs into three subsets (30): (I) a newly developed cancer (a cancer developing more than 10 years after subtotal gastrectomy for benign or malignant disease); (II) a recurrent cancer (a cancer developing on the anastomosis site less than 10 years after surgery for gastric malignancy); and (III) a residual cancer (a cancer developing less than 10 years after surgery for gastric malignancy except at the anastomosis site, or less than 10 years after benign gastric surgery). This classification is based on the time interval between subtotal gastrectomy and tumour recurrence, and also on the location of the tumor recurrence (at the anastomosis site or non-anastomosis site) in order to differentiate recurrent and primary cancers. There are characteristic RGC endoscopic findings according to the criteria of Kaminishi’s classification (Figures 3,4). In addition, these endoscopic characteristics could be partly explained by the pathologic differences. According to the UICC (International Union against Cancer) TNM criteria there were statistically significant differences in the T staging among three groups; residual cancers were less invasive than the recurrent and newly developed cancers (31). Furthermore, based on the Japanese research society of gastric cancer (JRSGC) classification, 81.8% (9/11) of the residual cancers were differentiated, whereas 14.3% (3/21) of the recurrent cancers and 30.4% (7/23) of the newly developed cancers were differentiated type adenocarcinoma (4,32). By Lauren’s classification, 90.0% (9/10) of residual cancers were intestinal type, while 89.5% (17/19) of the recurrent cancers and 66.7% (14/21) of the newly developed cancers were diffuse type (3,4). These findings for the residual cancers (well differentiated and elevated type EGC) are very similar to the characteristics of synchronous multiple gastric cancers (33). The prevalence of multiple gastric cancers is between 3.7-6.7% (7,34). Multiple carcinomas were associated significantly more often with adenomas, atrophic gastritis or IM than that of the solitary carcinomas (34). It is suggested that multiple and solitary carcinomas represent different developmental stages of a fundamentally identical process, with the cancer phenotype being dependent on the speed of progression. In other words, slow progression results in multiple tumors and rapid progression results in solitary tumors. Therefore, the residual cancers might have originated from those multiple gastric cancers that were not detected during the initial workup. Advanced RGCs were more easily detected in recurrent cancers than in residual cancers (4,5). These lesions might have resulted from the downward growing nature and undifferentiated pathology of the recurrent cancer.
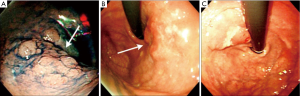
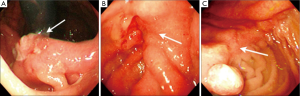
Surgery for gastric carcinoma and anastomotic recurrence
It is a highly selective group of patients in which curative surgery can be offered after excluding metastatic disease. Patients appropriate for a laparotomy after pre-operative staging are those with lesions that appear to be confined to the stomach and the peri-gastric lymph nodes (N1 and N2). The type of gastrectomy required depends on the position of the cancer and the margin necessary to be certain not to leave malignant cells at the anastomotic line. For an antral carcinoma which are mostly of the intestinal type, a subtotal gastrectomy with D2 (2nd tier) systematic lymphadenectomy and gastroenterostomy is deemed curative. A total D2 gastrectomy and Roux-en Y oesophago-jejunostomy for a more proximal gastric tumour gives better loco-regional clearance (1).
Park et al. reported that amongst RGCs that developed less than 10 years after surgery at the anastomosis site were depressed type cancers according to the Japanese classification system. Although the remnant stomach after benign gastric surgery is considered as a risk factor for the development of gastric cancer, it takes a longer time for obvious reasons (35,36). The exact mechanism is unclear but achlorhydria, atrophic gastritis, previous gastric surgery, an N-nitroso compound, persistent bile reflux, denervation of the gastric mucosa and bacterial invasion of the gastric stump have been regarded as possible etiologies (37-39). Among them, the combination of persistent bile reflux and denervation are assumed to enhance tumorigenesis at the anastomosis site (39). Therefore, those patients who had undergone Billroth II reconstruction at the initial surgery for benign lesions tended to have the development of cancer at stump site, whereas those patients who undergone Billroth I tended to have cancer at the non-stump site (40). Almost all the RGC patients (13/14) with initially benign disease developed gastric cancer on the anastomosis site, except in one case. In contrast, only 65% (5/9) of newly developed cancer patients with initially malignant diseases developed cancer at the stump site. This result shows that the gastric mucosa of the patients who underwent malignant gastric surgery tends to develop cancer more easily than those patients with initially benign disease. Endoscopic surveillance should therefore not concentrate only on the anastomotic site as residual cancers may be missed.
Conclusions
Survival following curative surgery (R0) for early gastric cancer has increased and, therefore showed the need for endoscopic surveillance for local recurrence. Most recurrent cancers occur at the anastomosis site, are advanced and of the diffuse type. Most residual cancers are of intestinal type and also occur in non-anastomosis sites. Incomplete IM (type III) with sialomucins and sulphomucins staining demonstrated in the resected specimen is the appropriate IM that requires subsequent endoscopic follow up. Endoscopic surveillance should not only be focused on the anastomotic site as in benign disease but also on the rest of the gastric remnant. By attenuating the chronic gastritis-metaplasia-dysplasia-carcinoma sequence, the role of H. pylori eradication therapy in preventing anastomotic recurrence or a new primary, should not be underestimated.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med 1995;333:32-41. [PubMed]
- Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer, 2010.
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965;64:31-49. [PubMed]
- Park JH, Lee JH, Rhee PL, et al. Endoscopic Screening for Remnant Gastric Cancer: Points to be Considered. Gut Liver 2007;1:22-6. [PubMed]
- Hosokawa Y, Konishi M, Sahara Y, et al. Limited subtotal gastrectomy for early remnant gastric cancer. Gastric Cancer 2014;17:332-6. [PubMed]
- Johannesson KA, Hammar E, Staël von Holstein C. Mucosal changes in the gastric remnant: long-term effects of bile reflux diversion and Helicobacter pylori infection. Eur J Gastroenterol Hepatol 2003;15:35-40. [PubMed]
- Greene FL. Management of gastric remnant carcinoma based on the results of a 15-year endoscopic screening program. Ann Surg 1996;223:701-6; discussion 706-8. [PubMed]
- Rokkas T, Filipe MI, Sladen GE. Detection of an increased incidence of early gastric cancer in patients with intestinal metaplasia type III who are closely followed up. Gut 1991;32:1110-3. [PubMed]
- de Vries AC, Haringsma J, de Vries RA, et al. The use of clinical, histologic, and serologic parameters to predict the intragastric extent of intestinal metaplasia: a recommendation for routine practice. Gastrointest Endosc 2009;70:18-25. [PubMed]
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [PubMed]
- Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer 2007;110:2119-52. [PubMed]
- Correa P, Cuello C, Duque E. Carcinoma and intestinal metaplasia of the stomach in Colombian migrants. J Natl Cancer Inst 1970;44:297-306. [PubMed]
- de Vries AC, Kuipers EJ. Epidemiology of premalignant gastric lesions: implications for the development of screening and surveillance strategies. Helicobacter 2007;12 Suppl 2:22-31. [PubMed]
- Wu X, Chen VW, Andrews PA, et al. Incidence of esophageal and gastric cancers among Hispanics, non-Hispanic whites and non-Hispanic blacks in the United States: subsite and histology differences. Cancer Causes Control 2007;18:585-93. [PubMed]
- Fennerty MB, Emerson JC, Sampliner RE, et al. Gastric intestinal metaplasia in ethnic groups in the southwestern United States. Cancer Epidemiol Biomarkers Prev 1992;1:293-6. [PubMed]
- Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am 2002;11:235-56. [PubMed]
- Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol 2010;105:493-8. [PubMed]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992;52:6735-40. [PubMed]
- Watari J, Das KK, Amenta PS, et al. Effect of eradication of Helicobacter pylori on the histology and cellular phenotype of gastric intestinal metaplasia. Clin Gastroenterol Hepatol 2008;6:409-17. [PubMed]
- Gutiérrez-González L, Wright NA. Biology of intestinal metaplasia in 2008: more than a simple phenotypic alteration. Dig Liver Dis 2008;40:510-22. [PubMed]
- Heilmann KL, Höpker WW. Loss of differentiation in intestinal metaplasia in cancerous stomachs. A comparative morphologic study. Pathol Res Pract 1979;164:249-58. [PubMed]
- Teglbjaerg PS, Nielsen HO. “Small intestinal type” and “colonic type” intestinal metaplasia of the human stomach, and their relationship to the histogenetic types of gastric adenocarcinoma. Acta Pathol Microbiol Scand A 1978;86A:351-5. [PubMed]
- Silva E, Teixeira A, David L, et al. Mucins as key molecules for the classification of intestinal metaplasia of the stomach. Virchows Arch 2002;440:311-7. [PubMed]
- Jass JR, Filipe MI. The mucin profiles of normal gastric mucosa, intestinal metaplasia and its variants and gastric carcinoma. Histochem J 1981;13:931-9. [PubMed]
- Matsukura N, Suzuki K, Kawachi T, et al. Distribution of marker enzymes and mucin in intestinal metaplasia in human stomach and relation to complete and incomplete types of intestinal metaplasia to minute gastric carcinomas. J Natl Cancer Inst 1980;65:231-40. [PubMed]
- Con SA, Con-Wong R, Con-Chin GR, et al. Serum pepsinogen levels, Helicobacter pylori CagA Status, and cytokine gene polymorphisms associated with gastric premalignant lesions in Costa Rica. Cancer Epidemiol Biomarkers Prev 2007;16:2631-6. [PubMed]
- Miki K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer 2006;9:245-53. [PubMed]
- Helsingen N, Hillestad L. Cancer development in the gastric stump after partial gastrectomy for ulcer. Ann Surg 1956;143:173-9. [PubMed]
- Balfour DC. factors influencing the life expectancy of patients operated on for gastric ulcer. Ann Surg 1922;76:405-8. [PubMed]
- Kaminishi M, Shimoyama S, Yamaguchi H, et al. Classification and carcinogenesis of gastric stump cancer. Gastroenterol Surg 1993;16:1253-65.
- Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 1997;80:1803-4.
- Japanese Research Society for Gastric Cancer. Japanese classification of gastric carcinoma. 1st English edition. Tokyo: Kanehara & Co., Ltd., 1995.
- Wittekind C, Klimpfinger M, Hermanek P, et al. Multiple simultaneous gastric carcinomas. Br J Cancer 1997;76:1604-9. [PubMed]
- Kodera Y, Yamamura Y, Torii A, et al. Incidence, diagnosis and significance of multiple gastric cancer. Br J Surg 1995;82:1540-3. [PubMed]
- Fisher SG, Davis F, Nelson R, et al. A cohort study of stomach cancer risk in men after gastric surgery for benign disease. J Natl Cancer Inst 1993;85:1303-10. [PubMed]
- Lundegårdh G, Adami HO, Helmick C, et al. Stomach cancer after partial gastrectomy for benign ulcer disease. N Engl J Med 1988;319:195-200. [PubMed]
- Pointner R, Schwab G, Königsrainer A, et al. Gastric stump cancer: etiopathological and clinical aspects. Endoscopy 1989;21:115-9. [PubMed]
- Northfield TC, Hall CN. Carcinoma of the gastric stump: risks and pathogenesis. Gut 1990;31:1217-9. [PubMed]
- Kaminishi M, Shimizu N, Shiomoyama S, et al. Etiology of gastric remnant cancer with special reference to the effects of denervation of the gastric mucosa. Cancer 1995;75:1490-6. [PubMed]
- Tanigawa N, Nomura E, Niki M, et al. Clinical study to identify specific characteristics of cancer newly developed in the remnant stomach. Gastric Cancer 2002;5:23-8. [PubMed]


