HER2-positive, trastuzumab-resistant metastatic esophageal cancer presenting with brain metastasis after durable response to dual HER2 blockade: a case report
Introduction
Gastroesophageal cancer is the second most common cause of cancer-related death in the world (1). Despite the use of combination chemotherapy regimens as first-line therapy, the prognosis for metastatic esophageal cancer remains poor, with median overall survival (OS) less than 1 year (2). Trastuzumab, a monoclonal antibody targeting human epithelial growth factor receptor 2 (HER2), combined with chemotherapy, was demonstrated to prolong OS to 13.8 months in the recent ToGA study (3). In the United States, chemotherapy in combination with trastuzumab has become the new standard of care for patients with gastroesophageal cancer overexpressing HER2.
Here we report a case of an advanced HER2-positive esophageal cancer, which responded well to trastuzumab-containing chemotherapy initially for 1 year. However, the patient developed liver metastases indicating the tumor’s resistance to trastuzumab. The therapy was switched to capecitabine with dual HER2 blockade comprising of both trastuzumab and lapatinib, and the patient responded remarkably well with resolution of liver metastases. Later, she developed HER2-negative brain metastases and local progression of disease.
Case report
A 55-year-old Caucasian woman with a past medical history of acid reflux for 7 years presented with substernal chest pain for two weeks in September, 2011. She underwent esophagogastroduodenoscopy (EGD), which revealed an ulcerated circumferential mass at the distal esophagus. Biopsy confirmed adenocarcinoma. Further workup with positron emission tomography-computed tomography (PET/CT) scan showed stage IVa disease with a hypermetabolic mass in the distal esophageal area as well as hypermetabolic lymph nodes involving the left supraclavicular region, left upper paratracheal region, celiac region, portocaval region and left periaortic region (Figure 1A). She was treated initially with folinic acid, fluorouracil, oxaliplatin (FOLFOX) for two cycles prior to coming to our center. HER2 testing of primary tumor was positive by fluorescent in-situ hybridization (FISH), therefore her treatment was switched to taxotere, carboplatin and herceptin (TCH). From January to April, 2012, she received four cycles of TCH which she tolerated very well. Repeat PET-CT in February, 2012 showed radiographic complete remission (data not shown). After discussion at multi-disciplinary tumor board, she was started on concurrent radiation to the primary tumor and previous lymphadenopathy sites along with weekly carboplatin, paclitaxel and trastuzumab. Her treatment course was complicated by neutropenic fever after 4 weekly doses of chemotherapy. As a result, carboplatin and paclitaxel were discontinued, and she finished the remainder of the radiation therapy concurrently with trastuzumab only. After concurrent chemoradiation, her treatment was continued with maintenance trastuzumab.
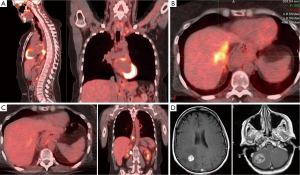
In October, 2012, a PET-CT showed liver metastasis (Figure 1B). Based on preclinical studies and clinical data from breast cancer, she was treated with capecitabine combined with dual HER2 blockade consisting of both trastuzumab and lapatinib (4,5). Once again, she tolerated well. In January, 2013 PET-CT showed resolution of liver metastasis and stable distal esophageal lesions (Figure 1C).
Unfortunately, in April, 2013, she suddenly developed severe occipital headache with nausea, vomiting, diplopia and ataxia and was taken to an emergency room immediately. MRI brain revealed an approximately 3 cm mass in the right cerebellum with an adjacent 4 mm lesion, and a 1.8 cm mass in the right parietal lobe. Both large lesions were associated with vasogenic edema resulting in partial effacement of the fourth ventricle and the right lateral ventricle, respectively (Figure 1D). She underwent craniotomy with resection of both right cerebellar and parietal tumors to achieve immediate decompression. Pathology confirmed metastatic adenocarcinoma consistent with the patient’s known history of an esophageal carcinoma. Interestingly, HER2 testing was negative. Unfortunately, the surgery was complicated by CSF fistula with pseudomeningocele in the right posterior fossa and right cerebellar abscess and right suboccipital epidural abscess requiring further surgical intervention. Due to these complications, she only received 1,800 cGy (200 cGy/fraction) out of a total of initially planned dosage of 3,000 cGy for whole brain radiation. In July and September, 2013, repeat MRI showed a 5.2 mm lesion in right cerebellum, and a 3.5 mm lesion in the right temporal lobe, compatible with metastases. Both lesions were treated successfully with 1-fraction stereotactic radiosurgery. Despite the fact that she did not receive any systemic therapy for over 4 months, she did not have any evidence of progression of extracranial disease until August, 2013.
In September, 2013, she started to experience progressively worsening dysphagia and odynophagia, and PET-CT showed increased fludeoxyglucose (FDG) uptake in the distal esophagus, but without any evidence of distal metastasis. EGD demonstrated local progression of her disease and repeat esophageal tumor biopsy confirmed HER2 overexpression by FISH, and therefore her chemotherapy with capecitabine, trastuzumab and lapatinib was resumed. Genomic alteration analysis by Foundation One (approximately over 200 most common cancer-related mutations were tested) revealed acquired mutations in both brain metastases and in the re-biopsy site of the local recurrence as compared with the first biopsy of the primary tumor biopsy. Clinically, her dysphagia continued to progress on treatment with worsening odynophagia requiring higher dose of narcotics, and brachytherapy was recommended to palliate her symptoms. However, the patient’s performance status started to deteriorate progressively, hence hospice was recommended. She died a few weeks later.
Discussion
Approximately 24% of gastroesophageal adenocarcinomas overexpress HER2, which has been associated with more aggressive biological behavior and poor outcome (6). The combination of trastuzumab with chemotherapy resulted in a 47% objective response rate as compared to 34% with chemotherapy alone, and also prolonged the median OS of gastroesophageal cancer significantly from under 12 to 13.8 months (3). Unfortunately, approximately 50% of patients did not exhibit an objective response—a condition commonly referred as de novo resistance. For those patients who did initially exhibit an objective response, most eventually developed disease progression while on trastuzumab therapy—a condition is commonly referred as acquired resistance (7). The development of resistance in gastroesophageal cancer is strikingly similar to that in breast cancer (8). Consequently, the median progression-free survival (PFS) achieved in the trastuzumab plus chemotherapy arm was merely 6.7 months (3). Our patient responded to HER2-based therapy for 12 months before she developed liver metastases-acquired resistance to trastuzumab. Preclinical studies suggest a diverse array of potential mechanisms of resistance to trastuzumab. Alterations in the HER2 receptor complex are proposed to be one of mechanisms involved in resistance. The decreased expression level of HER2, overexpression of other HER-family proteins such as HER3 or high level of intrinsic p95 HER2 protein are all associated with decreased response to HER2-directed therapy (7). Downstream activation of HER 2 signaling pathways, in particular, phosphatidylinositol 3-kinase (PI3K) pathway activation is another potential mechanism. Loss of the tumor suppressor phosphatase and tensin homolog (PTEN), which is a negative regulator of the PI3K pathway, active mutation in catalytic subunit (PIK3CA) of PI3K, and activation of AKT have all been shown to cause resistance to anti-HER2 therapy. Despite our better understanding of resistance mechanisms, it remains challenging to treat HER2-positive gastroesophageal cancer with trastuzumab resistance. HER2-positive breast cancer appears to continue to derive benefit from trastuzumab in combination with a different chemotherapeutic agent beyond disease progression on first-line trastuzumab-based chemotherapy (9,10). Currently, there is no high-level evidence to guide clinical practice regarding continuation of trastuzumab in gastroesophageal cancer in the presence of disease progression. Lapatinib, a potent tyrosine kinase dual inhibitor for both HER1 and HER2, has been demonstrated to be an effective agent against trastuzumab-resistant metastatic breast cancer when combined with either trastuzumab or capecitabine (4,11). Currently both regimens have been used as second-line therapy for metastatic breast cancer in the US. Combinations of chemotherapy with both trastuzumab and lapatinib showed significantly higher pathological complete remission (pCR), a surrogate marker for OS, than with chemotherapy plus trastuzumab alone in several neoadjuvant trials in early breast cancer (12). Lapatinib also exhibited a synergistic effect with trastuzumab against HER2-positive gastroesophageal cancer cell lines and xenografts (5). Based on these data, our patient was treated with capecitabine combined with both trastuzumab and lapatinib when she developed liver metastases on trastuzumab. She tolerated treatment well and responded rapidly with resolution of her liver lesions (Figure 1C), and remained in remission for totally 6 months. Further randomized studies are needed to validate the efficacy of this combination in a large population of patients.
Our patient also developed brain metastases, requiring emergent surgical intervention and subsequent radiation. Brain metastasis secondary to esophageal cancer is regarded as a rare event, with a reported incidence ranging from 1-3% in early case series (13,14). In contrast, up to 50% of stage IIIA non-small-cell lung cancer patients experienced brain metastasis at 3 years after initial therapy (15). Moreover, lung adenocarcinoma appears to correlate with a higher incidence of brain metastasis compared with squamous cell carcinoma (15,16). In a large cohort of patients with esophageal cancer, approximately 2% of patients (27 out of 1,588) had a diagnosis of brain metastasis. Interestingly, majority (82%) of patients had adenocarcinoma, suggesting a similar trend (14). The predominant histology of esophageal cancer has shifted from squamous cell carcinoma to adenocarcinoma in the past few decades. Nevertheless, its impact on the incidence of brain metastasis remains unknown. Most of the literature regarding brain metastasis was based on the outcome of patients with esophageal cancer treated in 1980s and 1990s when current effective systemic therapy and modern diagnostic imaging were not available. More recent reports seem to suggest that the incidence is becoming higher, ranging from 6-13%, which might reflect the advances in both systemic therapy and diagnostic imaging (17,18). As a matter of fact, none of the reports specifically looked at the cumulative incidence during the course of treatment in patients presenting with metastatic esophageal cancer other than brain metastasis, such as in our patient, but rather a cohort of patients in mixed stages presenting with brain metastases during follow-up. Such reports quite possibly underestimated the true incidence of brain metastases in metastatic esophageal cancer. HER2-positive breast cancer is well-known to have a substantially increased incidence of brain metastases (19). As HER2-positive esophageal cancer is a recently recognized entity of disease, the question of whether HER2 overexpression is associated with an increased incidence of brain metastases has not been prospectively studied. One retrospective case series suggests a similar association as in breast cancer (20). With further development of more effective HER2-directed therapy, the cumulative incidence of brain metastases in the metastatic setting would be expected to increase.
Previously, we reported a case of heterogeneity of HER2 expression in three distinctive fields of the primary tumor in a patient with metastatic esophageal tumor, which might be responsible for resistance (21). In the present case, we demonstrated HER2-negative brain metastases after 16 months of HER2-directed therapy. This is similar to what is seen in HER2-breast cancer, which can become HER2-negative following treatment with trastuzumab (22). Such discordance of HER2 expression between primary tumor and metastatic lesions after trastuzumab exposure will have a significant impact on the selection of systemic chemotherapy, especially anti-HER2 therapy, in metastatic esophageal cancer. Interestingly, genomic alteration analysis in brain metastases as well as in local recurrence by Foundation One revealed multiple acquired mutations involving PI3K pathway other than the biologically conserved mutations in the primary tumor (Table 1). Although the whole cancer genome analysis is not available, our case seems to illustrates this unique biological phenomenon-intratumor heterogeneity (acquired mutation and loss of HER2 expression), likely due to the impact of selective pressure secondary to systemic therapy and it has undoubtedly contributed to drug resistance and treatment failure. Orchestrated efforts are required to better understand and overcome intratumor heterogeneity in esophageal cancer in the future.

Full table
In summary, we report a case of HER2-positive esophageal adenocarcinoma with acquired resistance to trastuzumab-based therapy. Dual HER2 blockade appeared to be very effective. Unfortunately, the patient died from brain metastases and local recurrence eventually. Intratumor heterogeneity and brain metastasis are discussed, and future clinical trials are needed to address these important issues in esophageal cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- American Cancer Society. Cancer Facts & Figures 2011. Atlanta, USA, 2011.
- Howlader N, Noone AM, Krapcho M, et al. eds. SEER Cancer Statistics Review, 1975-2008. Bethesda, MD: National Cancer Institute, 2011.
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [PubMed]
- Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 2010;28:1124-30. [PubMed]
- Wainberg ZA, Anghel A, Desai AJ, et al. Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo. Clin Cancer Res 2010;16:1509-19. [PubMed]
- Tanner M, Hollmén M, Junttila TT, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol 2005;16:273-8. [PubMed]
- Stern HM. Improving treatment of HER2-positive cancers: opportunities and challenges. Sci Transl Med 2012;4:127rv2.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [PubMed]
- von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03-05 study. J Clin Oncol 2009;27:1999-2006. [PubMed]
- Petrelli F, Barni S. A pooled analysis of 2618 patients treated with trastuzumab beyond progression for advanced breast cancer. Clin Breast Cancer 2013;13:81-7. [PubMed]
- Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355:2733-43. [PubMed]
- Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 2012;379:633-40. [PubMed]
- Go PH, Klaassen Z, Meadows MC, et al. Gastrointestinal cancer and brain metastasis: a rare and ominous sign. Cancer 2011;117:3630-40. [PubMed]
- Weinberg JS, Suki D, Hanbali F, et al. Metastasis of esophageal carcinoma to the brain. Cancer 2003;98:1925-33. [PubMed]
- Mamon HJ, Yeap BY, Jänne PA, et al. High risk of brain metastases in surgically staged IIIA non-small-cell lung cancer patients treated with surgery, chemotherapy, and radiation. J Clin Oncol 2005;23:1530-7. [PubMed]
- Bajard A, Westeel V, Dubiez A, et al. Multivariate analysis of factors predictive of brain metastases in localised non-small cell lung carcinoma. Lung Cancer 2004;45:317-23. [PubMed]
- Smith RS, Miller RC. Incidence of brain metastasis in patients with esophageal carcinoma. World J Gastroenterol 2011;17:2407-10. [PubMed]
- Kanemoto A, Hashimoto T, Harada H, et al. Occurrence and clinical features of brain metastasis after chemoradiotherapy for esophageal carcinoma. J Radiat Res 2011;52:509-15. [PubMed]
- Gabos Z, Sinha R, Hanson J, et al. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol 2006;24:5658-63. [PubMed]
- Abu Hejleh T, Deyoung BR, Engelman E, et al. Relationship between HER-2 overexpression and brain metastasis in esophageal cancer patients. World J Gastrointest Oncol 2012;4:103-8. [PubMed]
- Niu J, Weber J, Gelbspan D. Change of HER2 status in metastatic esophageal adenocarcinoma: heterogeneity of the disease? Case report and review of literature. J Gastrointest Oncol 2012;3:358-61. [PubMed]
- Mittendorf EA, Wu Y, Scaltriti M, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res 2009;15:7381-8. [PubMed]


