A case of periampullary adenocarcinoma in neurofibromatosis type 1
Introduction
Neurofibromatosis type 1 (NF-1), also known as von Recklinghausen’s disease, is an autosomal-dominant disorder affecting males and females equally with an incidence of approximately one in 3,000 births (1,2). The classic clinical findings include café-au-lait spots, cutaneous neurofibromas, cognitive impairment, axillary and/or inguinal freckling, Lisch nodules (pigmented hamartomatous nevus of the iris) and bony abnormalities. In addition to these findings, patients with NF-1 also have an increased incidence of both benign and malignant neoplasms with the most common sites including the nervous system, skin, muscles and gastrointestinal tract.
Malignancies have been found in 3% to 15% of patients with NF-1 and are usually derived from neural-crest cells (2,3). Approximately 10-25% of patients with NF-1 have tumors involving the gastrointestinal tract with up to 95% patients being asymptomatic (4). When patients do present with symptoms it is usually related to the location of the tumor and they are most commonly found to have weight loss, abdominal pain, gastrointestinal bleeding, anemia and jaundice (2,4). The most common gastrointestinal tract tumors in patients with NF-1 include gastrointestinal stromal tumors (GISTs) of the small intestine, hyperplasia of the intestinal myenteric/submucosal plexuses and periampullary carcinoid tumors (somatostatin-producing carcinoids) (4). The periampullary region includes the duodenum, ampulla of Vater, distal biliary tract and head of the pancreas (5).
Adenocarcinomas (ACs) of the gastrointestinal tract have also been reported in patients with NF-1, however it is significantly less common compared to the other tumor types (4). There have been limited reports of gastrointestinal tract ACs in the esophagus, stomach, small bowel, gallbladder, liver, pancreas and colon (2). Periampullary ACs are one of the least common tumors found in patients with NF-1, with limited case reports in the literature (2-4,6,7). We report a unique presentation of periampullary AC in a NF-1 patient who presented with abdominal pain and obstructive jaundice.
Clinical presentation
A 56-year-old woman with history of NF-1 presented with a five-day history of intermittent right upper quadrant (RUQ) abdominal pain and one-day history of dark urine and scleral icterus. On exam, she had scleral icterus, mild RUQ abdominal pain with negative Murphy’s sign and diffuse cutaneous neurofibromas. Her admission labs are included in Table 1 and were significant only for hyperbilirubinemia.
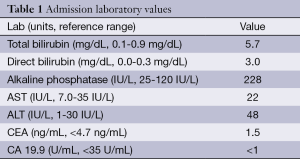
Full table
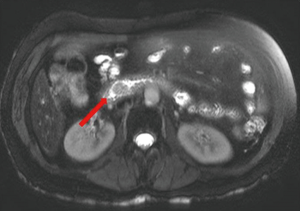
Computed tomography (CT) of the abdomen and pelvis showed an ill-defined 8 mm × 8 mm area of hypoattenuation in the ampullary region along with cholelithiasis and intra- and extrahepatic biliary ductal dilatation. MRI/MRCP of the abdomen showed an enhancing soft tissue mass in the periampullary region (Figure 1), 16 mm in diameter along with persistent biliary ductal dilatation and a small filling defect in the distal common bile duct consistent with choledocholithiasis.
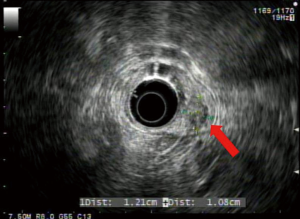
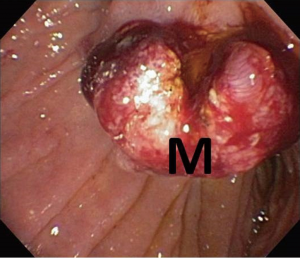
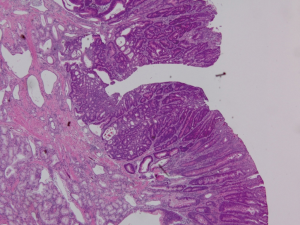
Endoscopic ultrasound (EUS) showed a 12 mm × 11 mm hypoechoic heterogeneous mass at the ampulla that was entirely confined to the superficial and deep mucosal layers (Figure 2). Endoscopic retrograde cholangiopancreatography (ERCP) showed the same periampullary mass (Figure 3) and the patient underwent biopsy, sphincterotomy and extraction of stones through the common hepatic duct and common bile duct. Biopsies of the periampullary mass showed adenoma with high grade dysplasia (HGD) and the patient was referred for surgery. Several weeks later after her initial presentation, the patient underwent a pylorus preserving pancreaticoduodenectomy (PPPD). On excision, the periampullary tumor was 2.5 cm × 1.9 cm × 1.1 cm and 5.5 cm from the proximal duodenal resection margin. Pathology showed well differentiated adenocarcinoma that arose in an adenoma (Figure 4) distal to the ampulla and within the duodenal mucosa of the papilla. There were no regional lymph node metastases. The pancreatic neck, uncinate, proximal duodenal, distal small bowel and bile duct margins were all negative for dysplasia and malignancy. Her cancer was classified as stage IA (pT1 N0 M0). Her post-operative course was complicated by a pancreaticojejunostomy leak that required insertion of an intra-abdominal drainage catheter removed within several weeks of placement. The patient was clinically stable and doing well at her one and two month follow-up appointments. She was scheduled to have follow-up CT scans of her abdomen and pelvis every 6 months for the next 2 years and then annually. No adjuvant chemotherapy is planned. At four months post-surgery, the patient had no evidence of recurrence.
Discussion
The most recent review of the literature conducted by Relles et al. in 2010 identified only six cases of periampullary adenocarcinoma in patients with NF-1 between 1989 and 2009 (4). A prior review performed by Costi et al. identified 12 cases (15 total tumors) of periampullary adenocarcinoma in patients with NF-1 between 1967 and 2001 (2). The overall incidence of periampullary carcinoma in the general population is also relatively low, with rates of 11.7, 0.88, 0.49 and 0.01 per 100,000 in the pancreas, bile duct, ampulla and duodenum respectively (8). Although periampullary carcinomas are a distinct group of malignancies that are separate from pancreatic ductal adenocarcinoma, they can clinically present similarly with abdominal pain, weight loss and jaundice. Most patients with NF-1 with gastrointestinal involvement are asymptomatic, however when there is periampullary involvement, patients can present early secondary to their symptoms associated with obstructive jaundice.
Based on the patient’s initial histopathological results obtained from ERCP that showed HGD along with the presence of symptoms secondary to the tumor, the decision to undergo PPPD was made. Jaundice in the setting of an ampullary mass is considered highly predictive of malignancy and therefore pancreaticoduodenectomy was preferred over endoscopic ampullectomy or local resection. The patient’s jaundice could have been due to her cholelithiasis, however the gallstones were relatively small and less likely to be the primary cause of her total bilirubin reaching 5.7 mg/dL. Endoscopic ampullectomy is an option for those patients believed to have benign ampullary tumors amenable to complete endoscopic removal (9). Surgical resection (e.g., local resection, Whipple procedure, PPPD) continues to be the first line treatment for patients with NF-1 with periampullary tumors (4). In patients with locally unresectable tumors, metastatic disease or who are poor surgical candidates, chemotherapy +/- radiation therapy are treatment options (9). The most commonly used chemotherapy agents include gemcitabine and 5-FU based regimens (9). After resection, patients may receive adjuvant chemotherapy and radiation, however level 1 evidence in favor of this approach is limited. The ESPAC-3 Periampullary Cancer Randomized Trial conducted by Neoptolemos et al. showed no survival benefit to using adjuvant fluorouracil or gemcitabine in periampullary adenocarcinoma patients after resection when compared to observation alone in patients with negative margins and negative lymph nodes (8). Based on the patient’s negative margins, early stage malignancy and available clinical data, no adjuvant chemotherapy is planned at the time of this publication. The genetic mutation of the NF1 gene on chromosome 17 (17q11.2) leads to a lower neurofibromin expression. The underlying pathophysiology of how this exactly predisposes patients with NF-1 to gastrointestinal tract tumors is not entirely clear, however neurofibromin is a tumor suppression gene that has been shown to be a negative regulator of the RAS pathway (4). Loss of neurofibromin activity leads to Ras activation, which in turn causes downstream activation of the AKT/mTOR and mitogen-activated protein kinase (MAPK) pathways causing cell proliferation (10). Activation of the Ras pathway is a common characteristic of adenocarcinoma of the gastrointestinal tract (11). There are an increasing number of clinical trials in patients with NF-1 that are evaluating the potential roles of medications such as everolimus, a mTOR inhibitor, that specifically target activated signaling due to NF1 inactivation (12).
Our case raises several interesting questions. There are no formal gastrointestinal cancer screening guidelines for patients with NF-1, regardless of the type of cancer. Since upper gastrointestinal tract tumors are common in patients with NF-1, it may be reasonable to consider performing upper endoscopic (e.g., EUS, ERCP, EGD) investigations to screen patients with NF-1. The exact percentages vary between studies, however up to 95% of patients with NF-1 with gastrointestinal tract tumors can be asymptomatic which would directly impact how to best to screen patients with NF-1 (4). The age at which to start screening patients with NF-1 would need to be established through more thorough clinical studies since there is limited data. Costi et al. showed that patients with NF-1 with periampullary adenocarcinoma presented at an earlier age (mean 47.1 years old, range 27 to 73) compared with patients that did not have NF-1 (60 years old), although the review was limited to 12 total patients (2). Prior case reports and series have also shown an association between periampullary ACs and additional periampullary tumors, which would also need to be incorporated into screening guidelines (2). Interestingly, our patient was 56 years old at presentation which is closer to the age of patients without NF-1 and did not have additional periampullary tumors. The best biomarker (e.g., CA 19.9, CEA) at time of diagnosis to assist in evaluating prognosis as well as for post-intervention monitoring remains to be defined. The fact that our patient had normal levels of both CA 19.9 and CEA may represent a positive prognostic indicator, however additional data is needed to further clarify the role of these biomarkers in patients with NF-1. Although the exact underlying pathogenesis of periampullary ACs in patients with NF-1 is not entirely understood, there could be a role for targeted chemotherapy such as everolimus in a patient such as ours if she recurred, in light of recent reports that mTOR inhibition is effective in other tumor types with NF-1 inactivation (12).
Conclusions
Periampullary ACs are rare in patients with NF-1, yet this pathologic diagnosis should be included in the differential in the presence of symptoms referable to the upper gastrointestinal tract in patients with NF-1.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Basile U, Cavallaro G, Polistena A, et al. Gastrointestinal and retroperitoneal manifestations of type 1 neurofibromatosis. J Gastrointest Surg 2010;14:186-94. [PubMed]
- Costi R, Caruana P, Sarli L, et al. Ampullary adenocarcinoma in neurofibromatosis type 1. Case report and literature review. Mod Pathol 2001;14:1169-74. [PubMed]
- Joo YE, Kim HS, Choi SK, et al. Primary duodenal adenocarcinoma associated with neurofibromatosis type 1. J Gastroenterol 2002;37:215-9. [PubMed]
- Relles D, Baek J, Witkiewicz A, et al. Periampullary and duodenal neoplasms in neurofibromatosis type 1: two cases and an updated 20-year review of the literature yielding 76 cases. J Gastrointest Surg 2010;14:1052-61. [PubMed]
- Klein A, Clemens J, Cameron J, et al. Periampullary neoplasms in von Recklinghausen’s disease. Surgery 1989;106:815-9. [PubMed]
- Yalagachin G, Mahantshetti P. Obstructive jaundice secondary to ampullary adenocarcinoma in neurofibromatosis type 1. Indian J Surg 2013;75:113-5. [PubMed]
- Jones TJ, Marshall TL. Neurofibromatosis and small bowel adenocarcinoma: an unrecognised association. Gut 1987;28:1173-6. [PubMed]
- Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA 2012;308:147-56. [PubMed]
- Romiti A, Barucca V, Zullo A, et al. Tumors of ampulla of Vater: A case series and review of chemotherapy options. World J Gastrointest Oncol 2012;4:60-7. [PubMed]
- Le LQ, Parada LF. Tumor microenvironment and neurofibromatosis type I: connecting the GAPs. Oncogene 2007;26:4609-16. [PubMed]
- Schönleben F, Qiu W, Allendorf JD, et al. Molecular analysis of PIK3CA, BRAF, and RAS oncogenes in periampullary and ampullary adenomas and carcinomas. J Gastrointest Surg 2009;13:1510-6. [PubMed]
- Endo M, Yamamoto H, Setsu N, et al. Prognostic significance of AKT/mTOR and MAPK pathways and antitumor effect of mTOR inhibitor in NF1-related and sporadic malignant peripheral nerve sheath tumors. Clin Cancer Res 2013;19:450-61. [PubMed]


