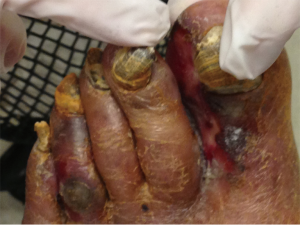
Bilateral above knee amputations after prolonged exposure to sorafenib and trebananib
Case presentation
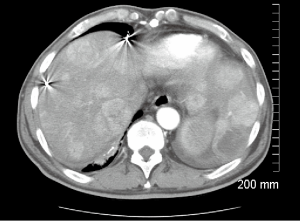
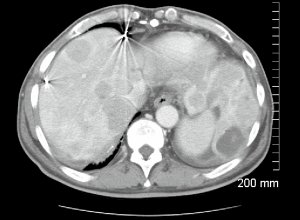
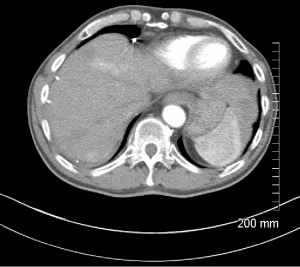
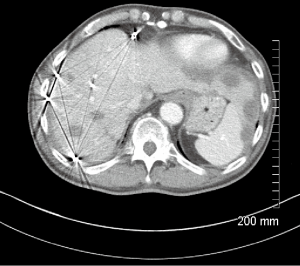
A 66-year-old male with a history of hepatitis C, and a 22 pack year (PY) smoking history, was referred to medical oncology for evaluation of unresectable and non-transplantable hepatocellular carcinoma (HCC). This was diagnosed after presenting to the emergency department with abdominal pain and a CT scan of the abdomen revealed innumerable hypervascular lesions ranging from 1.5-5 cm in the liver (Figures 1,2). AFP at presentation was 7.2 ng/mL. A liver biopsy was performed and pathology revealed moderately differentiated HCC. Patient was enrolled in a Phase two open label multicenter study to evaluate the efficacy and safety of trebananib (AMG386) an angiopoietin 1/2 neutralizing peptibody with potential antiangiogenic activity and standard dose of sorafenib as first line therapy for advanced or inoperable HCC. Patient did well on therapy other than developing weight loss warranting a reduction in trebananib dose which was subsequently well tolerated. Patient was followed with serial CT scans over a 3-year period revealing continued decrease in size of hepatic lesions, some necrosis and no progression of disease (Figures 3,4).
After 24 months on combination therapy, he complained of a 3-week history of left foot pain and paresthesia with an ulcer on that foot at a follow-up clinic visit (Figure 5). At that point both trebananib and sorafenib were held. He was evaluated by vascular surgery and underwent an angiogram which demonstrated patchy areas of stenosis throughout the superficial femoral and popliteal arteries bilaterally. He was also found to have a tight occlusion of the left proximal anterior tibial artery and posterior tibial artery. He had a balloon angioplasty done which restored flow in the left superficial femoral, popliteal and posterior tibial artery. Patient did not appear to take prescribed clopidogrel post procedure. He was seen in the oncology clinic the following week and found to have no distal pulses in his left foot as well as changes consistent with critical ischemia. He underwent an emergent left below knee amputation (L BKA) which was revised to a left above knee amputation (L AKA) 5 days later. This complication ultimately removed him from the trial. He was restarted on sorafenib 200 mg twice daily 2 months after the L AKA.
Eight months after initial ischemic event, he presented to the ER with right foot pain and changes consistent with right foot critical ischemia. Distal pulses could not be felt or identified by Doppler. He also had erythema with a blister extending to his distal medial shin. He ended up having a right BKA which was revised to an AKA after 4 days. Patient had been off sorafenib 1 week prior to right AKA and has remained off it since then. His most recent CT scans 36 months since initial diagnosis reveals no progression of disease (Figures 3,4).
Discussion
We report on a case of a patient with unresectable HCC with a 22 PY smoking history treated with trebananib and sorafenib who had a significantly longer than expected survival/PFS compared to the average HCC patient but ended up having bilateral AKA. This is the first report of such a case to the best of our knowledge.
Sorafenib is an oral tyrosine kinase inhibitor (TKI) that acts on many targets including RAF kinases, vascular endothelial growth factor (VEGF) 1,2,3, platelet derived growth factor and c-kit receptor. These kinases mediate tumor angiogenesis and inhibiting them prevents tumor growth. Both the SHARP trial and the Asia-Pacific Trial demonstrated that sorafenib improved survival and progression free survival in patients with advanced HCC and it is now considered first line therapy in patients with advanced unresectable HCC. Both trials did not report any events of peripheral vascular disease (1,2). Toxicities that have been frequently cited with TKIs include fatigue, diarrhea, mucositis, and hand-foot syndrome.
Other toxicities including hypertension (HTN) and arterial thromboses have also been reported (3-5). HTN the most common cardiovascular side effect was reported to be 5% in the SHARP trial and 18.8% in the Asia-Pacific trial (1,2). A recent meta-analysis revealed that patients on sorafenib had three times the risk of developing any grade of HTN and severe HTN (6). While there is no documented literature on peripheral arterial ischemia, there are previous reports of myocardial ischemia associated with TKIs. Based on clinical trials it has been estimated that 3% of patients had myocardial ischemia with sorafenib and up to 30% patients may have silent cardiovascular events (7-9). Case reports have also shown that sorafenib may induce coronary artery vasospasms (10,11).
Various mechanisms have been proposed for vascular ischemia associated with sorafenib and other TKIs. The VEGF are critical in endothelial cell regulation, by stimulating endothelial cell proliferation and inhibiting inflammation and apoptosis. It also plays a role in producing nitric oxide (NO) which vasodilates vessels, promotes both antithrombotic and anti-oxidant effects. These in conjunction allow the vessel to maintain its vascular architecture (12,13). Based on these properties it has been postulated that VEGF inhibitors disrupts vascular integrity as well as potentiate a deficiency of NO, thus creating a pro-thrombotic state. This is evidenced by a recent meta-analysis by Choueiri et al. which found that there was a 3-fold increased risk of arterial events amongst patients taking sorafenib compared to controls (4). Furthermore the study also showed that HTN may further precipitate arterial thromboses (4).
In our patient, it is important to note that he is a current smoker and has a 22 PY smoking history but had never complained of PVD symptoms prior to 24 months on the combination of sorafenib and trebananib. While it is difficult to say what exactly precipitated these adverse events, it is possible that he had long standing PVD that resulted in bilateral critical ischemia following prolonged exposure to one or both agents that in turn have also stabilized his cancer and prolonged his life.
To the best of our knowledge, critical limb ischemia has never been reported with sorafenib or trebananib.
Conclusions
This is the first reported case of bilateral BKA after being on sorafenib and trebananib therapy. While this is one report, other investigators experience with patients that have risk factors for PVD with prolonged exposure to dual antiangiogenic therapies would be useful in determining if aggressive risk factor management and close surveillance be advised when patients are placed on these therapies.
Acknowledgements
Disclosure: Olugbenga Olowokure is on the speakers bureau of celegene, Novartis and Bayer.
References
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [PubMed]
- Sonpavde G, Bellmunt J, Schutz F, et al. The double edged sword of bleeding and clotting from VEGF inhibition in renal cancer patients. Curr Oncol Rep 2012;14:295-306. [PubMed]
- Choueiri TK, Schutz FA, Je Y, et al. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol 2010;28:2280-5. [PubMed]
- Elice F, Rodeghiero F. Side effects of anti-angiogenic drugs. Thromb Res 2012;129 Suppl 1:S50-3. [PubMed]
- Li Y, Li S, Zhu Y, et al. Incidence and risk of sorafenib-induced hypertension: a systematic review and meta-analysis. J Clin Hypertens (Greenwich) 2014;16:177-85. [PubMed]
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125-34. [PubMed]
- Khakoo AY, Yeh ET. Therapy insight: Management of cardiovascular disease in patients with cancer and cardiac complications of cancer therapy. Nat Clin Pract Oncol 2008;5:655-67. [PubMed]
- Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 2008;26:5204-12. [PubMed]
- Naib T, Steingart RM, Chen CL. Sorafenib-associated multivessel coronary artery vasospasm. Herz 2011;36:348-51. [PubMed]
- Pantaleo MA, Mandrioli A, Saponara M, et al. Development of coronary artery stenosis in a patient with metastatic renal cell carcinoma treated with sorafenib. BMC Cancer 2012;12:231. [PubMed]
- González-Pacheco FR, Deudero JJ, Castellanos MC, et al. Mechanisms of endothelial response to oxidative aggression: protective role of autologous VEGF and induction of VEGFR2 by H2O2. Am J Physiol Heart Circ Physiol 2006;291:H1395-401. [PubMed]
- Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res 2001;49:568-81. [PubMed]