Stereotactic body radiation therapy for liver metastases
Background
Over the years, the concept of metastatic disease has evolved and the therapeutic approach has changed. In 1907 Halsted described his theory on the spread of breast cancer, perhaps the first modern theory on cancer metastasis. He proposed an orderly spatial and temporalprogression from primary site to locoregional lymphatics and ultimately to disseminated disease. Radical locoregional therapy was thought reduce the risk of metastatic disease by removing the source of metastases (1). Subsequently, other researchers, notably Dr. Bernard Fisher, who also studied breast cancer, proposed that metastasis was a systemic phenomenon, and that local therapy could do little to affect a patient’s survival once the cancer had metastasized (2). Since then, the historical concept of metastic disease as an incurable state for which only systemic therapies and palliative interventions are appropriate has been questioned. In the 1990’s Weichselbaum and Hellman formulated a new hypothesis, according to which the metastatic disease is exists as part of a “spectrum” of clinical states. The intermediate state, called “Oligometastatic disease”, represented a condition between the absence of metastases and widespread dissemination (3). Patients with oligometastatic disease might potentially enjoy extended survival or even be cured with aggressive local therapy to their metastatic tumor sites. Recent improvements in diagnostic imaging have allowed the early diagnosis of metastatic disease in a higher number of patients and nowadays the prevalence of patients in the oligometastatic state is increasing (4).
Colorectal cancer (CRC) is one of the tumors that most often presents with solitary or oligometastasic disease, commonly in the liver. It is estimated, indeed, that 30% to 70% of patients with CRC will develop liver metastases and in this subset, hepatic progression is an important cause of morbidity and mortality (5,6). In patients with untreated colorectal liver metastases the overall survival (OS) is about 31% at 1 year, 8% at 2 years, 3% at 3 years, and 1% at 4 years, with a median survival ranging from 6 to 12 months (7). The introduction of modern chemotherapy regimens in the current treatment for colorectal liver metastases has improved the progression free survival rate and to a lesser extent the OS, due tolimited local control (LC) of disease (8-10). Historically, surgical resection of CRC liver metastases improves OS, with 1 and 5-year rates of 90-95% and 30-60%, respectively and with a median OS of 40-53 months (11-14). In 1999, Fong et al. defined the main favorable prognostic factors for oligometastatic CRC patients treated with surgery of liver metastases and included the absence of extrahepatic disease, metachronous presentations with extended disease-free intervals (DFI) >12 months, single metastasis <5 cm, early-stage primary tumors, low carcinoembryogenic antigen (CEA) and negative hepatic surgical margins, suggesting that the LC of liver oligometastases can improve the systemic control of the disease, in selected patients (13). More recently, a published study by Tomlinson et al. showed an increase long-term cancer-specific survival at 10 years after resection, confirming the achievement of cure in this subset of oligometastatic CRC patients (15).
The role of ablation of non-CRC liver metastases is controversial. Many reports examined outcomes for patients with non-colorectal liver metastases treated with hepatic resection and initially showed that only neuroendocrine metastases subgroup had a better prognosis (16-19). A recent study analyzed the outcomes of 1,452 patients with limited liver metastases from non-CRC, non-neuroendocrine metastasis and concluded that “in current practice, liver surgery […] should be considered only when the metastatic disease is well controlled or responding to systemic therapy. When applied in these situations, surgery may be able to offer selected patients a real benefit in long-term survival (20).
However, only 10-20% of patients were suitable for surgical resection because of technical difficulties, unfavorable tumor factors or patient comorbidities (12,13).
In the last decade, minimally-invasive loco-regional approaches were introduced as an alternative to surgery, including radiofrequency ablation (RFA) or trans-catheter arterial chemo-embolization (TACE). The results in terms of LC and OS rates are promising, but these techniques present some limitations related to lesion size and location (lesions higher than 3 cm of diameter or in proximity of major blood vessels, the main biliary tract or the gallbladder, or just beneath the diaphragm) (20-24). An effective, safe and non-invasive alternative therapeutic option is necessary in the 60-80% of oligometastatic patients, who can benefit from locally ablative therapy of liver metastases, but probably never fit to surgery.
Stereotactic body radiation therapy (SBRT) as an alternative ablative treatment of liver metastases
Historically, radiation therapy has had a limited role in the treatment of liver metastases. The low tolerance of liver tissue to irradiation raises the risk of the radiation-induced liver disease (RILD). RILD syndrome is characterized by anicteric ascites with elevation of alkaline phosphatase and liver transaminases, which occurs two weeks to four months after radiotherapy and can results in liver failure and death (25). According to a radiobiological model, the liver has a parallel architecture; therefore the risk of RILD is proportional to the mean radiation dose delivered to normal liver tissue (25). Safe radiation treatment of liver metastases should be possible with a technique that delivers a very conformal radiation dose to the tumor and a minimal radiation dose to surrounding critical tissues. This technique is known as SBRT or stereotactic ablative radiotherapy (SABR). In contrast to conventional radiotherapy, which delivers low-dose fractions (ranging from 1.5-3 Gy) to a larger volume for a higher number of daily fractions, SBRT entails precise delivery of high-dose in a single or a few fractions (1 to 6 fractions), with tumor ablation and maximal normal-tissue sparing. Practice guidelines for the performance of SBRT were published in 2010 by ASTRO and ACR. To ensure the delivery accuracy, the target position is checked before or during SBRT treatment, by an integrated image acquisition system (image-guided radiation therapy or IGRT) (26,27).
SBRT for liver metastases: state of the art
In the last years, SBRT was used as non-invasive loco-regional treatment for many primary and secondary tumors, with promising results (28,29). Five retrospective (30-34) and eight prospective (35-41) studies have investigated the efficacy of SBRT in the treatment of liver metastases from various primary tumors. No randomized phase III data have been published. We reviewed in detail the heterogeneous prospective reports (Table 1), with regard to patients selection, dose prescription, toxicity and outcome, in terms of LC and OS.
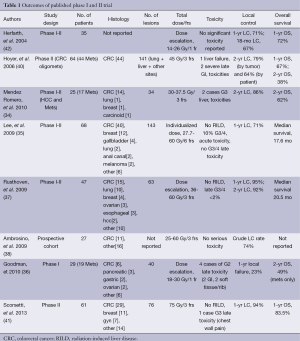
Full table
In all studies, liver metastases from colorectal, breast and lung cancer were most frequently treated, with a number less than 5 and maximum tumor size of 6 cm. Only two studies focusing on SBRT for liver metastases from a single primary tumor type (CRC) were published, including 44 and 20 treated patients respectively (39,40). Regardless of age, enrolled patients had a good performance status (Eastern Cooperative Oncology Group 0-1 or Karfnosky >70), with absentt or stable extrahepatic disease and adequate hepatic volume and function.
Prescription doses, generally ranged from 30 to 60 Gy in 3 fractions, although in one published phase II trial a higher prescription dose of 75 Gy in 3 fractions was delivered (41). In two prospective studies a single fraction of 14-30 Gy was employed (35,36) and in one phase I trial an individualized radiation doses ranging from 27.7 to 60 Gy in 6 fractions was delivered (35).
Most studies showed a low toxicity profile with a ≥ G3 toxicity rate of 1-10% and the incidence of RILD less than 1%. Lee et al. (35), did not experience RILD in 68 patients treated with 6 fractions and median liver dose of 16.9 Gy (range, 3-22 Gy). In a phase I/II study by Rusthoven et al. (37) on 47 patients and in a phase II trial by Scorsetti et al. (41) on 61 patients, no RILD was observed using a dose constraint allowing no more than 700 mL of uninvolved liver to receive 15 Gy or greater in 3 fractions. The most common G2 toxicities included a transient hepatic transaminase increase within 3 months of SBRT (39,41) and gastrointestinal, soft-tissue and bone complications, related to lesions close to the duodenum, bowel, skin and ribs. Duodenal ulceration and intestinal perforation were observed in 3 patients with maximum doses greater than 30 Gy in 3 fractions to the duodenum and bowel (40). One case of Grade 3 soft-tissue toxicity was observed for dose of 48 Gy in 3 fractions to a subcutaneous tissue (37). In 2 patients, nontraumatic rib fractures were experienced for maximum doses of 51.8 Gy and 66.2 Gy in 6 fractions to 0.5 cm3 of rib (35). One patient suffered from chronic chest wall pain G3, which resolved within 1 year after SBRT for a prescription dose of 75 Gy in 3 fractions (41).
LC rates varies from 70% to 100% at 1 year and 60% to 90% at 2 years and correlated to lesion size <3 cm, as showed by Rusthoven et al. (37) and to higher prescription dose, as suggested by Lee et al. (35). In a phase II study, Scorsetti et al. revealed no significantly increased risk of local recurrence for lesion diameter >3 cm compared with smaller metastases, using an ablative prescription dose of 75 Gy in 3 fractions (41).
Two-years OS rate was 30-83%, with a median OS rate ranging from 10 to 34 months. OS correlation with lesion size higher than 3 cm and metachronous presentation for CRC liver metastases was demonstrated by Hoyer et al. (40).
SBRT for liver metastases: practice guideline
Patient selection
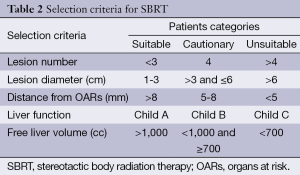
Selection criteria for patients with liver metastases candidate to SBRT are controversial and a multidisciplinary tumor board discussion is recommended. Figure 1 shows the patient’s treatment algorithm. According to the current literature, patients who may be candidates for SBRT can be separated into three categories: suitable, cautionary and unsuitable patients. Table 2 shows the characteristics for all groups.
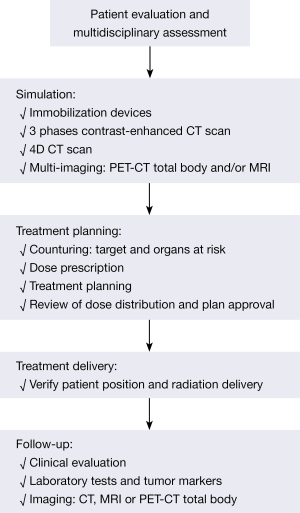
Full table
The best candidates for SBRT are oligometastatic patients with a good performance status (Eastern Cooperative Oncology Group 0-1 or Karfnosky >70), controlled or absent extra-hepatic disease, number of hepatic lesions ≤3, size lesions ≤3 cm, lesion distance from organs at risk (OARs) >8 mm, good liver function (Childs A) and healthy liver volume >1,000 cc. In patients with 4 liver metastases, diameter ranging from 3 to 6 cm, OARs distance between 5 and 8 mm, moderate liver function (Child B) and healthy liver volume ranging from 700 to 1,000 cc, the use of SBRT must be evaluated with caution. Patients with ≥5 hepatic lesions, diameter greater than 6 cm, OARs distance less than 5 mm, inadequate liver function (Child C) and healthy liver volume <700 cc, are unsuitable for SBRT. Histopathology is not considered an inclusion or exclusion criteria and in several studies patients with different radioresistant and radiosensitive primary tumor were treated with similar LC rates. Similarly, age is not a selection criteria. SBRT, indeed, is a non-invasive and safe therapy ideal for elderly patients, who are often unsuitable for surgery.
Treatment planning and delivery
SBRT requires highly precise dose planning and delivery. In the Simulation phase, the patient is immobilized with a personalized device to ensure maximal accuracy and reproducibility of treatment. An abdominal compression device should be used to reduce the organ motion related to respiratory excursion (41). The latter can be evaluated by 4D-CT scan. A contrast-free computed tomography (CT) scan and a three-phase contrast-enhanced CT scan are acquired. Multi-modal imaging with contrast-enhanced Magnetic resonance imaging (MRI) and/or positron emission tomography (PET) is useful for better target definition. The clinical target volume (CTV) is defined as equal to the gross tumor volume (GTV). In all patients who underwent 4D-CT scan, an internal target volume (ITV) is defined as the envelope of all GTVs in the different respiratory phases. The planning target volume (PTV) is generated from either the GTV or the ITV by adding margin to compensate for the uncertainties of set-up and organ motion (43). Treatment planning SBRT requires a highly conformal dose distribution, with multiple beams using either coplanar or non-coplanar geometries. Intensity modulated RT (IMRT) and, more recently, Volumetric Arc Radiation Therapy (VMAT) improves the homogeneous distribution of dose around concave targets and reduces irradiation of OARs (26,27). Patient position check is necessary before each treatment session. Image guided radiation therapy (IGRT) should be performed before each daily session to reduce set-up uncertainties. Fiducial markers are employed for target localization in selected patients. Percutaneous fiducial implantation is a mildly-invasive procedure with a related risk of seeding and migration (44). Surgery was administrated in 40-50% of patients treated with SBRT in some series and in these patients, surgical clips can be used as fiducial markers (41).
Dose prescription and OARs dose constraints
An ablative dose prescription is related to LC rates (40,45). For SBRT in 3 fractions, a prescription dose greater than 48 Gy should be considered, if granted by normal tissue constraints (46). A total dose of 60 Gy is recommended for lesions with a diameter ≤3 cm (37), while for lesions with a diameter >3 cm a higher prescription dose is necessary to obtain similar LC (41) (Table 3).

Full table
Recommended dose constraints for OARs are listed in Table 4 (37,41). In case of overlap between the PTV and gastrointestinal tract or heart, the priority should be given to OARs (41).
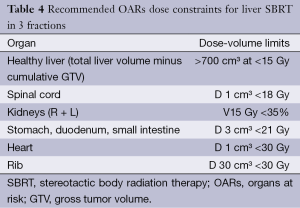
Full table
Response evaluation
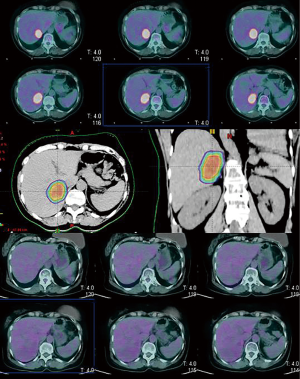
Assessment of tumor response can be performed based on European Organization for Research and Treatment of Cancer Response Evaluation Criteria In Solid Tumors (EORTC-RECIST) criteria (45) and MRI can be used during the follow up especially for hepatic lesions poorly detected on CT. The radiographic response after SBRT are typically slow, as demonstrated by Herfath et al. which showed the progressive radiographic response and the characteristic reduction of focal liver reaction after 2-4 months and 11-15 months respectively, in our series (42). If PET-CT is performed before SBRT, it is recommended to employ PET Response Criteria in Solid Tumors (PERCIST) (47), as showed in Table 5. Figure 2 shows the complete metabolic response (CMR) in a single hepatic lesion after SBRT.

Full table
Future directions
The role of liver SBRT for metastatic disease needs to be evaluated by randomized clinical trials comparing the various alternative therapies.
Combination with chemotherapy and targeted therapy could be investigated to reduce the incidence of extra-field recurrences that are seen in a significant number of patients post SBRT. Selection of patients with favorable prognosis and further studies on histopathology-specific treatment to evaluate SBRT impact on survival is recommended.
Conclusions
SBRT is a non-invasive, well-tolerated and effective treatment for patients with liver metastases not suitable to surgical resection. Prospective randomized clinical trials are required to confirm clinical evidence and long term results.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Halsted WS. I. The Results of Radical Operations for the Cure of Carcinoma of the Breast. Ann Surg 1907;46:1-19. [PubMed]
- Fisher B. Laboratory and clinical research in breast cancer--a personal adventure: the David A. Karnofsky memorial lecture. Cancer Res 1980;40:3863-74. [PubMed]
- Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol 2011;8:378-82. [PubMed]
- Alongi F, Arcangeli S, Filippi AR, et al. Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist 2012;17:1100-7. [PubMed]
- Yoon SS, Tanabe KK. Surgical treatment and other regional treatments for colorectal cancer liver metastases. Oncologist 1999;4:197-208. [PubMed]
- Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: Impact of surgical resection on the natural history. Br J Surg 1990;77:1241-6. [PubMed]
- Bengmark S, Hafstrom L. The natural history of primary and secondary malignant tumors of the liver. I. The prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer 1969;23:198-202. [PubMed]
- Saltz LB. Metastatic colorectal cancer: is there one standard approach? Oncology (Williston Park) 2005;19:1147-54; discussion 1154, 1157-8, 1160.
- Cunningham D, Pyrhonen S, James RD, et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 1998;352:1413-8. [PubMed]
- Chang AE, Schneider PD, Sugarbaker PH, et al. A prospective randomized trial of regional versus systemic continuous 5-fluorodeoxyuridine chemotherapy in the treatment of colorectal liver metastases. Ann Surg 1987;206:685-93. [PubMed]
- Cummings LC, Payes J, Cooper G. Survival after hepatic resection in metastatic colorectal cancer: A population-based study. Cancer 2007;109:718-26. [PubMed]
- Wei AC, Greig PD, Grant D, et al. Survival after hepatic resection for colorectal metastases: A 10-year experience. Ann Surg Oncol 2006;13:668-76. [PubMed]
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18. [PubMed]
- Leonard GD, Brenner B, Kemeny NE. Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J Clin Oncol 2005;23:2038-48. [PubMed]
- Tomlinson JS, Jarnagin WR, Dematteo RP, et al. Actual 10-year survival after resection of 514 colorectal liver metastases defines cure. J Clin Oncol 2007;25:4575-80. [PubMed]
- Berney T, Mentha G, Roth AD, et al. Results of surgical resection of liver metastases from non-colorectal primaries. Br J Surg 1998;85:1423-7. [PubMed]
- Lindell G, Ohlsson B, Saarela A, et al. Liver resection of noncolorectal secondaries. J Surg Oncol 1998;69:66-70. [PubMed]
- Benevento A, Boni L, Frediani L, et al. Result of liver resection as treatment for metastases from noncolorectal cancer. J Surg Oncol 2000;74:24-9. [PubMed]
- van Ruth S, Mutsaerts E, Zoetmulder FA, et al. Metastasectomy for liver metastases of non-colorectal primaries. Eur J Surg Oncol 2001;27:662-7. [PubMed]
- Adam R, Chiche L, Aloia T, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg 2006;244:524-35. [PubMed]
- de Meijer VE, Verhoef C, Kuiper JW, et al. Radiofrequency ablation in patients with primary and secondary hepatic malignancies. J Gastrointest Surg 2006;10:960-73. [PubMed]
- Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol 2009;19:1206-13. [PubMed]
- Siperstein AE, Berber E, Ballem N, et al. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg 2007;246:559-65; discussion 565-7. [PubMed]
- Fiorentini G, Aliberti C, Mulazzani L, et al. Chemoembolization in colorectal liver metastases: the rebirth. Anticancer Res 2014;34:575-84. [PubMed]
- Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 2002;53:810-21. [PubMed]
- Potters L, Kavanagh B, Galvin JM, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2010;76:326-32. [PubMed]
- Seung SK, Larson DA, Galvin JM, et al. American College of Radiology (ACR) and American Society for Radiation Oncology (ASTRO) Practice Guideline for the Performance of Stereotactic Radiosurgery (SRS). Am J Clin Oncol 2013;36:310-5. [PubMed]
- Høyer M, Swaminath A, Bydder S, et al. Radiotherapy for liver metastases: a review of evidence. Int J Radiat Oncol Biol Phys 2012;82:1047-57. [PubMed]
- Nair VJ, Pantarotto JR. Treatment of metastatic liver tumors using stereotactic ablative radiotherapy. World J Radiol 2014;6:18-25. [PubMed]
- Blomgren H, Lax I, Naslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995;34:861-70. [PubMed]
- Wada H, Takai Y, Nemoto K, et al. Univariate analysis of factors correlated with tumor control probability of three-dimensional conformal hypofractionated high-dose radiotherapy for small pulmonary or hepatic tumors. Int J Radiat Oncol Biol Phys 2004;58:1114-20. [PubMed]
- Wulf J, Guckenberger M, Haedinger U, et al. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol 2006;45:838-47. [PubMed]
- Katz AW, Carey-Sampson M, Muhs AG, et al. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys 2007;67:793-8. [PubMed]
- van der Pool AE, Méndez Romero A, Wunderink W, et al. Stereotactic body radiation therapy for colorectal liver metastases. Br J Surg 2010;97:377-82. [PubMed]
- Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol 2009;27:1585-91. [PubMed]
- Goodman KA, Wiegner EA, Maturen KE, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys 2010;78:486-93. [PubMed]
- Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009;27:1572-8. [PubMed]
- Ambrosino G, Polistina F, Costantin G, et al. Image-guided robotic stereotactic radiosurgery for unresectable liver metastases: Preliminary results. Anticancer Res 2009;29:3381-4. [PubMed]
- Méndez Romero A, Wunderink W, van Os RM, et al. Quality of life after stereotactic body radiation therapy for primary and metastatic liver tumors. Int J Radiat Oncol Biol Phys 2008;70:1447-52. [PubMed]
- Hoyer M, Roed H, Traberg HA, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol 2006;45:823-30. [PubMed]
- Scorsetti M, Arcangeli S, Tozzi A, et al. Is stereotactic body radiation therapy an attractive option for unresectable liver metastases? A preliminary report from a phase 2 trial. Int J Radiat Oncol Biol Phys 2013;86:336-42. [PubMed]
- Herfarth KK, Debus J, Wannenmacher M. Stereotactic radiation therapy of liver metastases: Update of the initial phase-I/ II trial. Front Radiat Ther Oncol 2004;38:100-5. [PubMed]
- ICRU. ICRU Report 83: Prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT). J ICRU 2010;10:1-106.
- Hennessey H, Valenti D, Cabrera T, et al. Cardiac embolization of an implanted fiducial marker for hepatic stereotactic body radiotherapy: a case report. J Med Case Rep 2009;3:140. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [PubMed]
- Chang DT, Swaminath A, Kozak M, et al. Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer 2011;117:4060-9. [PubMed]
- Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 2009;50 Suppl 1:122S-50S. [PubMed]


