Radioembolization of hepatic tumors
Background
Radioembolization (RE) is a form of brachytherapy during which microspheres containing Yttrium-90 (90Y) are implanted into hepatic tumors via the hepatic artery. The radiation is permanently bound to the microspheres, which do not migrate out of the liver tumors. Almost pure beta radiation is delivered within an effective range of only 2.5 mm from the microsphere, thus sparing normal adjacent liver tissue from damage. The half-life is 64 hours with all of the effective radiation delivered by 14 days post implant (1-3) (Figures 1-3).
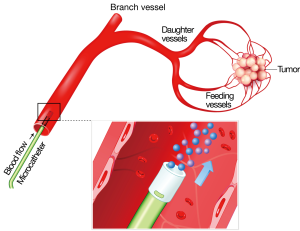
The liver is one of the most commonly involved organs in metastatic disease, second only to the lymph nodes (4). Solid tumors, mostly adenocarcinomas (29.5%) and neuroendocrine tumors (NET) (7.2%) account for the most commonly detected tumors in the liver, followed by hepatocellular carcinoma (HCC) (28%) (5). Due to the hematogenous nature of liver metastases, a high proportion (71%) of patients with gastrointestinal solid tumors present with liver metastases. Notably, however, liver metastases are also frequently detected among patients with neuroendocrine gastrinoma or glucagonomas (~40% of patients), melanoma (10-20% of patients with stage IV disease) as well as breast (~30% of patients), lung (~16% of patients) and kidney adenocarcinomas (~18% of patients) (4,6,7). Nevertheless, few tumor types, except colorectal cancer metastases (mCRC), carcinoid metastases, and HCC, present with lesions confined to the liver. For most tumor types, the presence of hepatic tumors is a sign of more disseminated disease (4,8) and a poor prognosis (9,10). For colorectal carcinoma, it is the presence or absence of liver metastases, rather than the primary carcinoma, that leads to significant clinical morbidity and determines the life expectancy for the patient (11).
A prospective evaluation of some 1,325 patients with colorectal cancer, conducted between January 1994 and December 1999, found that median survival following the diagnosis of colorectal liver metastases was approximately 10 months (range 4.6 to 23.1 months), with both the age of patient and the extent of disease being the key limiting factors for surgical resection and palliative chemotherapy (12).
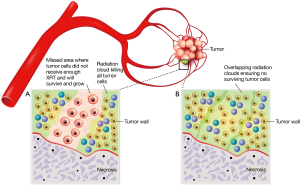
Key to the rationale of using particles for hepatic-artery treatment of cancer is the underlying therapeutic advantage provided by the predominant arterial supply to tumors versus the portal venous blood supply to normal liver parenchyma (13-15). Since 1971, with the now landmark observations of Dr. Judah Folkman, it has been recognized that to grow larger than 2 mm in diameter tumors must successfully recruit new blood vessels (16). Tumor vasculature composition was further detailed in this era by Ackerman et al., who estimated that a plexus of abnormal vessels, of up to 200 times the density of the vasculature in normal liver tissue, clusters around tumors (13-15). Therefore release of radioactive microspheres into the hepatic arteries selectively delivers them to the tumors instead of normal parenchyma. Contemporaneously, Folkman noted that tumors recruited this plexus of vessels by diffusible factors and proteins, which were ultimately termed vascular endothelial growth factors (VEGF) (17).Transmembrane receptors on the cell surface were subsequently discovered and shown to be involved with cancer cell proliferation, blockage of apoptosis, activation of tumor cell invasion and metastases, and stimulation of tumor-related neovascularization. These receptors were termed endothelial grow factor receptors (EGFR) (18). Currently, anti-VEGF therapy is mostly performed using bevacizumab, a human monoclonal immunoglobulin G1 (IgG1) antibody that selectively binds serum VEGF, inhibiting binding of VEGF to the cell surface receptor. Bevacizumab concentrates selectively in tumor tissues compared with normal tissues (19,20). Two anti-EGFR agents are used in mCRC treatment. The first to be used was cetuximab, a human mouse chimeric monoclonal IgG1 antibody that binds the extracellular portion of the EGFR receptor, blocking activation of intracellular pathways to cancer cell proliferation and tumor-induced neovascularization. The second approved agent was panitumumab, a fully human IgG2 monoclonal antibody that has the same action as cetuximab (18).
Radiation devices and clinical work up
Two commercial RE products are available for RE: resin microspheres and glass microspheres, both which use 90Y as the therapeutic agent. Because each type of microsphere differs in its composition, the amount of radiation carried per microsphere differs; however the procedure itself is the same for both. Resin microspheres (SIR-Spheres; Sirtex Medical, Sydney, Australia) are approved in the European Union, Asia and other countries for the treatment of unresectable liver tumors, and in the United States for the treatment of unresectable mCRC of the liver in combination with floxuridine hepatic arterial chemotherapy. In the USA, glass 90Y microspheres (TheraSphere; MDS Nordion, Ottawa, Canada) have been given a Humanitarian Device Exception from the Food and Drug Administration (FDA) for the treatment of unresectable HCC. In Europe and other countries, glass 90Y microspheres are approved for the treatment of unresectable liver tumors. To date, more than 40,000 treatments have been performed with 90Y-labeled microspheres (both resin and glass) in hepatic solid tumors. The technique of RE involves an outpatient procedure in which a transfemoral catheterization is performed and millions of radioactive microspheres (15-20 million resin; 1-8 million glass) are selectively released under fluoroscopic guidance into the hepatic arterial supply (21).
90Y is a near-pure beta emitter with half-life of 64.2 hours (94% of the energy is emitted in the first 11 days) and average tissue penetration of 2.5 mm. Radiation protection and isolation is not needed after implantation. The small size (25 to 45 microns) of radioactive microspheres does not produce significant ischemic effect as opposed to the larger than 100-micron particles used in chemoembolization. In summary, as with all radiation therapy treatments, a planning session or ‘simulation’ is essential for successful patient outcomes. During the week prior to treatment, each patient undergoes hepatic angiography to map the hepatic arterial system and protectively embolize any vessels that would permit microspheres to enter the GI tract (22). During the same procedure a mixture of albumin particles approximating the size of microspheres (25-35 microns) are bound to the gamma-emitting radioisotope technetium-99m (99mTc). After the procedure the albumin particles are imaged via single photon emission computed tomography (SPECT) gamma camera scintigraphy to detect shunting into the pulmonary vasculature or GI tract. A week later, patients return for placement of a microcatheter in the hepatic artery and delivery of microspheres that will preferentially embolize in the terminal arterioles of the tumors while sparing adjacent normal liver. The treatment team calculates the planned activity of 90Y for treatment to the whole liver or to only one lobe or segment as required for an individual patient. This multidisciplinary team, with complementary skills, includes experts from interventional radiology, radiation oncology, nuclear medicine, medical physics, diagnostic radiology, surgical oncology, and medical oncology (23,24).
At first consideration of RE it is not obvious that the release of microparticles from an intraarterial catheter placed into a branch of the hepatic artery feeding a tumor, will effectively deliver those particles to only the tumor. However, preclinical, clinical and histopathology proof substantiates that most of the radioactive microspheres do indeed become preferentially and permanently implanted in the tumor as opposed to normal liver (1,25-30).
Mechanism of action, dosimetry, radiobiology
Beta radiation causes the same type of tumor cell injury as other radioactive isotopes and external beam radiation, namely DNA damage. The dose rate of radiation striking the tumor nucleus is dependent on distance from the microsphere, and dramatically falls off beyond 2.5 mm. In many instances RE appears to be very effective because the microspheres are embedded within or very close to the tumor itself. Kennedy et al. performed microdosimetry calculations in 3D on tumor samples of a patient receiving RE. At 2 cm in diameter, the tumor nodule was completely encompassed by the 100 Gy isodose line, with significant areas within the tumor receiving a total dose of more than 1,000 Gy (1). It should be noted that RE delivers continuous radiation at a fraction of the dose rate provided by an intense but brief daily pulse with external beam radiation of photon X-rays. The radiobiology of the two types of radiation differs, which makes the comparison of doses absorbed by each modality problematic. External beam radiotherapy treatment courses rarely deliver more than 70 Gy total dose to tumors, in part due to tolerance of surrounding normal tissues and in part because clinically, 90% local control of tumors is expected at that high a dose level.
Yorke et al. (31) studied in 14 patients whether predictions of normal tissue complication probability (NTCP) models for liver, which were based on clinical data from external beam therapy, were consistent with early clinical results using 90Y. The parallel architecture model was used with external beam parameters, and modeling of 90Y dose rate effects were compared with observed outcomes in 90Y therapy. The Lyman model was not used due to its known sensitivity to small high-dose regions. According to these authors, weaknesses of the analysis were lack of specific dosimetry, generalized assumptions of repair time of normal liver, and tumor and dose-rate effects. However, the parallel model predictions of the non-uniform dose distribution of 90Y were consistent with the observed lack of liver complications.
Cremonesi et al. (32) reported on 20 patients with liver malignancies who were prospectively treated with single or fractionated 90Y RE. Biologically effective dose (BED) calculations were obtained by estimating absorbed dose via medical internal radiation dosimetry (MIRD) formalism from SPECT 99mTc MAA scans. Although there were assumptions made as to repair times, which dramatically alter BED estimates, it is the first use of biologic parameters in an attempt to optimize 90Y therapy. Their study conclusions suggested multiple fractions of 90Y would enable dose escalation in tumor while maintaining acceptable normal liver toxicity.
Patient selection/eligibility
As with any therapy it is essential that only properly selected patients be subjected to the risk of treatment complications and toxicity. General guidelines borne of extensive clinical experience for RE is published in the Radioembolization Brachytherapy Oncology Consortium (REBOC) Consensus document (2). Relative contraindications include abdominal ascites, severe portal hypertension, prior external beam radiotherapy, and liver to lung shunt fraction estimated at >20%. Absolute contraindications include decompensated liver function, hepatic encephalopathy, functional liver reserve <700 cc, pregnancy, and uncorrectable liver shunt to GI track or lungs (2,3,22,24,33-36). As a refinement of the inclusion/exclusion criteria in the REBOC document, many advanced users have found that total bilirubin remains the most important pretreatment indicator of post-radiation success in RE. Whereas HCC patients can routinely gain benefit without excessive toxicity when the upper limit of eligibility for RE is 2.0 mg/dL, the same is not true for patients with metastatic tumors. Particularly in mCRC, the best results appear in patients with normal total bilirubin levels at the time of RE (37-41).
A number of nonradioactive ablative therapies must be considered for small, limited-number hepatic tumors (radiofrequency ablation, cryotherapy, non-anatomical resection, irreversible electroporation) and specialized external beam radiation approaches including stereotactic body radiotherapy (SBRT) and proton beam therapy (PBT). A limited number of reports also cite good outcomes with the use of percutaneous brachytherapy techniques employing high-dose rate 192Ir afterloading sources (42). Our multidisciplinary GI tumor board often debates which of these many modalities we should use, and in which sequence. It is indeed fortunate that we have such a wealth of options to maximize chances of a successful individualized treatment approach to liver tumors. It has been our experience when considering the overlap of indications of most local ablative therapies, that in the majority of patients, due to the presence of numerous bilobar tumors, that intraarterial therapies [RE, and in HCC, transarterial chemoembolization (TACE)] are the best options available. A typical sequence for metastatic tumors is first, surgical resection, if possible; and if not, best local ablative treatment for 1-3 lesions, which often includes SBRT. For patients with metastatic lesions and HCC, as well as those with more than 3 tumors or geographically challenging tumor locations, RE is our standard therapy choice.
Outcomes in specific tumor types
Metastatic colorectal carcinomas (mCRC)
There have been more patients treated with mCRC treated with RE than any other disease type worldwide, and nearly all having received two or more lines of chemotherapy first. Correspondingly, a relatively larger publication record exists for RE in mCRC patients compared to all other types of cancers combined. Only a few key studies will be covered here. The history of RE approval in the US and elsewhere is nonstandard in that no phase I/II dose finding studies were performed for any tumor type; rather, only a few small experiences have been reported separately for glass and resin spheres. In 2001, however, a randomized controlled phase III study was completed by Gray et al. for resin sphere RE performed in patients with liver-only mCRC, with concurrent floxuridine (FUDR) given by hepatic artery infusion (43). These authors concluded that a single treatment with RE and FUDR in first line therapy of mCRC patients with liver-only disease was more effective in response rate and progression free survival than FUDR alone (15.9 vs. 9.7 mo., P=0.001). Rapid advances in chemotherapy and biologic agents for mCRC became available shortly after this study was completed making the study approach obsolete. In 2007 Sharma et al. (44) completed a formal phase I study of resin sphere RE and modern chemotherapy (FOLFOX4) as initial therapy for mCRC with liver-dominant disease. Safety was proven and an international follow-up study was completed (SIRFLOX, results pending) of more than 500 patients treated with FOLFOX6 and optional bevacizumab, combined with a single resin RE treatment during cycle 1, week 1 of therapy. The continuation of this worldwide study is called FOXFIRE, and will continue enrollment to a total of 1,100 patients; data will be combined with SIRFLOX data. First public results are expected in 2015 (45).
Second line therapy patients with hepatic mCRC have been studied with concurrent irinotecan in phase II fashion with encouraging results and no increase in toxicity over RE alone. In patients who had previously received 5-fluorouracil (5-FU) and either irinotecan or oxaliplatin (46% of patients) or both (27% of patients), monotherapy with resin RE was safe and produced a significant response with improved overall survival compared to published chemotherapy-only trials of similar patients (46,47). Mulcahy et al. in a phase II study of glass sphere RE treated a variety of mCRC patients with liver-dominant disease that had completed one, two and some three or more lines of chemotherapy (41). They achieved remarkable median overall survival in second line patients of 23.5 months; ECOG performance status 0 patients also reached a median survival of 23.5 months vs. only 6.7 months for ECOG 1 and 4 months for ECOG 2 (P≤0.0001).
Third line (and higher) chemorefractory patients with mCRC are often referred to as ‘salvage’ patients because no standard of systemic therapy exists for them, and they comprise the majority of patients seen and treated with RE. Hendlisz et al. (48) using resin sphere RE completed a phase III randomized controlled trial in mCRC patients with liver-only disease. The control arm was continuous infusion 5FU (300 mg/m2 D1-14 q3 weeks) until progression, and the experimental arm added resin RE on day 1, cycle 1 combined with 5FU protracted IV infusion (225 mg/m2 D1-14; 300 mg/m2 q3 weeks thereafter). A total of 46 patients were enrolled, all who had previously progressed on at least three lines of chemotherapy. The RE patients experienced less grade 3 or 4 toxicity (1 patient vs. 6 patients), and 10 patients in the control arm were allowed to cross over to the RE arm at time of progression. The RE patients achieved a significant increased time to liver progression (5.5 vs. 2.1 months, P=0.003); and time to any progression (5.6 vs. 2.1 months, P=0.03) (48). Because of allowed crossover, differences in overall survival between the two arms are not possible to distinguish.
In a complementary experience to that of Hendlisz, Cosimelli et al. (49) reported a multi-center Italian National Cancer Center prospective phase II study of chemotherapy-refractory mCRC patients with liver-dominant disease who had failed FOLFOX and FOLFIRI previously. A single resin RE treatment was delivered as monotherapy in 50 patients total and all were followed closely with imaging and physical exams. Of note, this patient cohort had extensive disease with 60% of the patients found to have 50-70% tumor burden replacing the liver parenchyma. Seventy percent of patients had bilobar disease, with median tumor size 5 cm (0.8-10 cm diameter). Radiographic response rate (RECIST 1.0) was 24%, with maximum response seen at 6 weeks post RE (range, 6-12 weeks). This is an earlier timeframe compared to most other studies with imaging response typically maximum at 10-14 weeks post RE. Median survival of the whole cohort was 12.6 months (95% CI: 7.0-18.3 months), with a median 16.0 months in responders [complete response (CR) + partial response (PR) + stable disease (SD)] (13.0-19.0 months) and non-responders only 8.0 months (range, 4-12.0 months) which was significant (P=0.0006) between all three (49).
The largest study to date in any patient group receiving RE has been in mCRC, third line or higher patients, reported by Kennedy et al. and the MORE study group (37-41,50-52). In an effort to learn more about treatment patterns, toxicity and outcomes after resin microsphere RE in the US, this multicenter collaboration retrospectively collected data on every patient with a diagnosis of mCRC treated at their institution since the beginning of their programs. An independent clinical research organization visited each of the 11 centers and collected data from the original source documents. An independent central radiology assessment by a radiology group outside the US, which is expert in RE patients, assigned a RECIST score at 3-month post-RE images. A total of 606 patients were studied with remarkably similar outcomes to previously published prospective studies and most retrospective studies in terms of toxicity, response, and overall survival. All 606 patients (370 males; 236 females) were studied with a median follow-up of 8.5 months (IQR 4.3-15.6) after RE. Fewer than 11% of patients were treated outside recommended guidelines, with grade 2 albumin (<3-2.0 g/dL) being the most common (10.5%) at time of RE. Key findings of the MORE authors included an extremely low rate (0.6%) of radiation related liver damage leading to liver failure. Radiation GI ulcers occurred in less than 2% of patients, and all grade 3 toxicities reached only 6% by CTCae 3.0 criteria. Approximately 20% of patients underwent a second treatment; the highest number was four separate RE treatment courses. Elderly (70 years old) and very elderly (75 years old and higher) patients had the same and often lower toxicity scores than younger patients. Median 90Y activity administered was 1.18 GBq (IQR 0.55). RECIST response at 3 months (n=184 patients) was 9.8% PR (n=18), 72.3% SD [133] and 17.9% PD [33]; Disease Control Rate =82.1%. Peri-tumoral edema was documented in 33% (n=60); necrosis in 42% [79]; both in 22% [40] of cases, respectively. No significant differences in background characteristics between responders and non-responders were evident (P>0.05). RECIST response at 3 months predicted survival: PR median 13.9 months (95% CI: 9.2-30.3 months) vs. SD 11.0 (8.9-13.5) vs. PD 6.7 (5.5-8.1) (P=0.002). The authors noted caution is needed in interpreting CT/MRI scans at 3 months post RE due to peri-tumoral edema, and other artifacts in the image, which may lead to either underestimation of the true PR/SD or overestimation of PD, respectively. Positron emission tomography (PET) scans were not part of the analysis as there were too few in the dataset to analyze.
These encouraging results match very closely with other large retrospective data published on mCRC in hundreds of patients in total (34,53-57).
Hepatocellular carcinoma (HCC)
No randomized controlled trial comparing RE with others therapies has been published yet but good level 2 evidence can be compiled from large, well-characterized cohort series published in the last 5 years (58-63). By and large, RE has been mainly used for patients whose disease is unresectable and are also not considered good candidates for TACE, either those in the advanced stage due to symptoms or portal vein thrombosis (PVT) or those in the intermediate stage with very large tumors or extensive bilobar involvement. In these poor TACE candidates, a case-control study indicated that RE might improve survival compared to experimental therapies or best supportive care (16 vs. 8 months, P<0.05) (64). When analyzed by tumor stage, intermediate stage patients treated by RE reach a median survival of 16-18 months (60-62) which compares well with the median survival achieved by TACE. Broadly equivalent survivals have also been reported in retrospective analyses of single institutions although treatment selection and lead-time biases should be considered. For patients in the intermediate stage who fail to respond to TACE, the remaining treatment options are the antiangiogenic and antiproliferative targeted agent sorafenib or RE. The Sorafenib HCC Assessment Randomized Protocol (SHARP) was the pivotal phase III randomized controlled trial that proved that systemic agent sorafenib prolonged survival of HCC patients. The target population was patients not amenable for TACE including those in the advanced stage HCC and those in the intermediate stage that had progressed or were considered poor TACE candidates. In a subset analysis of the SHARP trial, survival in patients failing TACE was 11.9 and 9.9 months, respectively, for the sorafenib and placebo-treatment arms (65). By comparison, survival was 11.4 months for a subset of usual candidates for RE matching the SHARP criteria and 15.4 months in BCLC B patients failing TACE (62).
Sorafenib is the mainstay for treating advanced HCC, defined by the presence of vascular invasion, extrahepatic disease or deteriorated performance status in a patient with at least partially preserved liver function. As RE has no macroembolic effect (66), it can be safely applied to patients with PVT, and can offer a median survival in the range of 6-13 months (58,60-62), very similar to those reaching 6.5-10.7 months as reported in the phase III clinical trials of sorafenib in the same group of patients (67,68). Furthermore, in patients with only branch or segmental PVT, survival extends to 10-14 months (60,69,70). Due to this growing body of level 2 evidence, RE has found a place in the guidelines adopted by the European Society for Medical Oncology (ESMO), the European Society of Digestive Oncology (ESDO), and the National Comprehensive Cancer Network (NCCN), albeit not in the guidelines of the European Association for the Study of the Liver (EASL) and the European Organization for Research and Treatment of Cancer (EORTC), or the American Association for the Study of the Liver Diseases (AASLD).
The indications described above have been adopted as standard in many referral centers. Other interesting indications that are still considered investigational target the population with less advanced tumors (71). RE can induce complete necrosis in small (<3 cm) tumors, as shown in the analyses of 35 explanted livers (30). In patients with inoperable early stage HCC, a median time to progression as long as 25.1 months (95% CI: 8-27 months) has been reported (72) and this may provide a rationale for its use as a bridge to liver transplantation in an attempt to avoid dropping from the waiting list (73). The potential to induce intense tumor responses has allowed RE to be used as a downstaging therapy, to reduce the tumor burden within acceptable limits for liver transplantation, to render non-operable patients operable, or to simplify surgery. Downsizing from UNOS T3 to T2 was achieved more frequently with TARE than with TACE (58% vs. 31%, P=0.023 (74). Furthermore, atrophy of the irradiated lobe after RE and contralateral lobe hypertrophy as a result of the injection of a high activity of 90Y in a lobar hepatic artery, known as ‘‘radiation lobectomy’’, may be valuable in itself and certainly could contribute to resectability (75). In a smaller group of 21 UNOS T3 stage patients, 29% were downstaged and underwent surgical resection or liver transplantation, with a 3-year survival rate of 75% (76), which is comparable with the survival in patients with early stage disease who are treated radically at the time of diagnosis.
RE is generally well tolerated and a post-embolization syndrome like the one that appears after TACE is not common. Rare complications resulting from the irradiation of non-tumoral tissues include pneumonitis (77), cholecystitis (78), gastrointestinal ulcerations (79), and liver damage. Liver toxicity is the most challenging adverse event in HCC patients, as the majority of these tumors arise in cirrhotic livers, with some degree of reduced functional reserve. A variable incidence of liver decompensation including ascites (0-18%) or encephalopathy (0-4%) has been reported. The incidence of RE-induced liver disease (characterized by jaundice and ascites appearing 4-8 weeks after RE) in cirrhotic patients was 9.3% in the largest series so far reported (80-83).
Metastatic neuroendocrine tumors (mNET)
Liver metastases from NET originating in the foregut, midgut, and hindgut are a substantial clinical entity, causing direct and negative impact on overall survival. Unlike mCRC and HCC, mNET are a diverse group of malignancies that cannot be approached with the expectation of similar results after an intervention. However, all the literature to date confirms that when sufficient radiation dose is delivered, objective responses are consistently good. Prior to 2008 most published data of radiotherapy for NET is anecdotal from small external beam studies, but surprisingly the most robust studies of radiotherapy and NET to date are in RE (84-90).
Keeping in mind the heterogeneity of this disease and thus the challenge in defining cohorts of patients with the same pathologic classification of disease, the symptomatic response rate to intraarterial therapies, transarterial embolization (TAE), TACE and RE ranges from 39-95%, 1-18 months after treatment. Moreover, the majority of patients experience improvement of symptoms of hormonal syndromes or symptoms from disease burden. Yang et al. recently summarized an extensive review of the literature regarding hepatic intraarterial mNET therapies but did not include SBRT, radiofrequency ablation, non-anatomic resection, or other ablative interventions (91).
Pavel et al. recently published a report on consensus guidelines of the European Neuroendocrine Tumor Society (ENETS) for the management of liver and other distant metastases from neuroendocrine neoplasms (92). Although the report covers general management options for liver metastases, it was not intended to produce detailed levels of evidence for best hepatic therapy. Building on ENETs work, a multidisciplinary group of experts convened to specifically answer several key questions regarding management of mNET hepatic disease. Conclusions were presented to a larger group of experts who served as a jury and the group’s overall consensus opinion was based on published literature through December 2012. Among the final statements of the workgroup was that the quality and strength of literature on TAE, TACE and RE is insufficient to determine which modality is superior in producing response or impact on survival. However, RE’s advantages over TAE/TACE included reduced side effects and fewer treatments required to control tumors. The workgroup also recommended that RE could be substituted for TAE/TACE in the ENETs Consensus Guidelines for patients with either liver-only or limited extrahepatic disease burdens (93,94).
Conclusions
Optimal treatment decisions for management of hepatic malignancies—either primary or secondary—are most often made during discussions in multidisciplinary tumor conferences. It is within this context that whether and when to use RE is best decided taking into consideration all of the various modalities now available to treat liver lesions. Thus far, the highest level (level 1) medical evidence supporting the use of internal radiation via 90Y microspheres has been shown in mCRC patients who have received prior first- and second-line chemotherapy and have liver-dominant metastatic disease. However, in HCC and mNET, level IIa evidence is consistently reported around the world, attesting to how robust, safe and effective a treatment RE can be when appropriately applied by those in skilled oncology centers.
Acknowledgements
My institution has received grant funding for a clinical trial from Sirtex Medical.
Disclosure: The author declares no conflict of interest.
References
- Kennedy AS, Nutting C, Coldwell D, et al. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys 2004;60:1552-63. [PubMed]
- Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007;68:13-23. [PubMed]
- Kennedy AS, Salem R. Radioembolization (yttrium-90 microspheres) for primary and metastatic hepatic malignancies. Cancer J 2010;16:163-75. [PubMed]
- Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer 2006;106:1624-33. [PubMed]
- Kasper HU, Drebber U, Dries V, et al. Liver metastases: incidence and histogenesis. Z Gastroenterol 2005;43:1149-57. [PubMed]
- Ihse I, Persson B, Tibblin S. Neuroendocrine metastases of the liver. World J Surg 1995;19:76-82. [PubMed]
- Rose DM, Essne R, Hughes TM, et al. Surgical resection for metastatic melanoma to the liver: the John Wayne Cancer Institute and Sydney Melanoma Unit experience. Arch Surg 2001;136:950-5. [PubMed]
- Pentheroudakis G, Fountzilas G, Bafaloukos D, et al. Metastatic breast cancer with liver metastases: a registry analysis of clinicopathologic, management and outcome characteristics of 500 women. Breast Cancer Res Treat 2006;97:237-44. [PubMed]
- Rose DM, Essner R, Hughes TM, et al. Surgical resection for metastatic melanoma to the liver: the John Wayne Cancer Institute and Sydney Melanoma Unit experience. Arch Surg 2001;136:950-5. [PubMed]
- Adam R, Aloia T, Krissat J, et al. Is liver resection justified for patients with hepatic metastases from breast cancer? Ann Surg 2006;244:897-907; discussion 907-8. [PubMed]
- McMillan DC, McArdle CS. Epidemiology of colorectal liver metastases. Surg Oncol 2007;16:3-5. [PubMed]
- Leporrier J, Maurel J, Chiche L, et al. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg 2006;93:465-74. [PubMed]
- Ackerman NB, Lien WM, Kondi ES, et al. The blood supply of experimental liver metastases. I. The distribution of hepatic artery and portal vein blood to “small” and “large” tumors. Surgery 1969;66:1067-72. [PubMed]
- Lien WM, Ackerman NB. The blood supply of experimental liver metastases. II. A microcirculatory study of the normal and tumor vessels of the liver with the use of perfused silicone rubber. Surgery 1970;68:334-40. [PubMed]
- Ackerman NB, Lien WM, Silverman NA. The blood supply of experimental liver metastases. 3. The effects of acute ligation of the hepatic artery or portal vein. Surgery 1972;71:636-41. [PubMed]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000;6:389-95. [PubMed]
- Folkman J. Tumor angiogenesis. Adv Cancer Res 1974;19:331-58. [PubMed]
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med 2008;358:1160-74. [PubMed]
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011-27. [PubMed]
- Eichholz A, Merchant S, Gaya AM. Anti-angiogenesis therapies: their potential in cancer management. Onco Targets Ther 2010;3:69-82. [PubMed]
- Kennedy A, Coldwell D, Sangro B, et al. Radioembolization for the Treatment of Liver Tumors. Am J Clin Oncol 2012;35:91-9. [PubMed]
- Lewandowski RJ, Sato KT, Atassi B, et al. Radioembolization with 90Y microspheres: angiographic and technical considerations. Cardiovasc Intervent Radiol 2007;30:571-92. [PubMed]
- Coldwell D, Sangro B, Wasan H, et al. General selection criteria of patients for radioembolization of liver tumors: an international working group report. Am J Clin Oncol 2011;34:337-41. [PubMed]
- Kennedy A, Coldwell D, Sangro B, et al. Integrating radioembolization ((90)Y microspheres) into current treatment options for liver tumors: introduction to the international working group report. Am J Clin Oncol 2012;35:81-90. [PubMed]
- Basciano CA, Kleinstreuer C, Kennedy AS, et al. Computer modeling of controlled microsphere release and targeting in a representative hepatic artery system. Ann Biomed Eng 2010;38:1862-79. [PubMed]
- Kennedy AS, Kleinstreuer C, Basciano CA, et al. Computer modeling of yttrium-90-microsphere transport in the hepatic arterial tree to improve clinical outcomes. Int J Radiat Oncol Biol Phys 2010;76:631-7. [PubMed]
- Basciano CA, Kleinstreuer C, Kennedy AS. Computational fluid dynamics modeling of 90Y microspheres in human hepatic tumors. J Nucl Med Radiat Ther 2011;2:1.
- Kennedy A, Dezarn W, Weiss A. Patient specific 3D image-based radiation dose estimates for 90y microsphere hepatic radioembolization in metastatic tumors. J Nucl Med Radiat Ther 2011;2:1.
- Carson JP, Kuprat AP, Colby SM, et al. Detecting distance between injected microspheres and target tumor via 3D reconstruction of tissue sections. Conf Proc IEEE Eng Med Biol Soc 2012;2012:1149-52.
- Riaz A, Kulik L, Lewandowski RJ, et al. Radiologic-pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheres. Hepatology 2009;49:1185-93. [PubMed]
- Yorke ED, Jackson A, Fox RA, et al. Can current models explain the lack of liver complications in Y-90 microsphere therapy? Clin Cancer Res 1999;5:3024s-30s. [PubMed]
- Cremonesi M, Ferrari M, Bartolomei M, et al. Radioembolisation with 90Y-microspheres: dosimetric and radiobiological investigation for multi-cycle treatment. Eur J Nucl Med Mol Imaging 2008;35:2088-96. [PubMed]
- Dezarn WA, Cessna JT, DeWerd LA, et al. Recommendations of the American Association of Physicists in Medicine on dosimetry, imaging, and quality assurance procedures for 90Y microsphere brachytherapy in the treatment of hepatic malignancies. Med Phys 2011;38:4824. [PubMed]
- Kennedy AS, McNeillie P, Dezarn WA, et al. Treatment parameters and outcome in 680 treatments of internal radiation with resin 90Y-microspheres for unresectable hepatic tumors. Int J Radiat Oncol Biol Phys 2009;74:1494-500. [PubMed]
- Lau WY, Kennedy AS, Kim YH, et al. Patient selection and activity planning guide for selective internal radiotherapy with yttrium-90 resin microspheres. Int J Radiat Oncol Biol Phys 2012;82:401-7. [PubMed]
- Salem R, Parikh P, Atassi B, et al. Incidence of radiation pneumonitis after hepatic intra-arterial radiotherapy with yttrium-90 microspheres assuming uniform lung distribution. Am J Clin Oncol 2008;31:431-8. [PubMed]
- Kennedy AS, Ball D, Cohen SJ, et al. Pre-90Y hepatic radiotherapy hemoglobin and liver functions to predict overall survival in unresectable, chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2014;32:abstr 292.
- Kennedy AS, Ball D, Cohen SJ, et al. Safety and efficacy of 90y resin microspheres in elderly (≥70 years) compared to younger patients with colorectal liver metastases (mCRC). J Clin Oncol 2013;31:abstr e14545.
- Kennedy AS, Ball D, Cohen SJ, et al. Hepatic imaging response to 90Y-microsphere therapy administered for tumor progression during systemic chemotherapy in patients with colorectal liver metastases. J Clin Oncol 2013;30:abstr 270.
- Kennedy AS, Ball D, Cohen SJ, et al. U.S. patients receiving resin 90Y microspheres for unresectable colorectal liver metastases: A multicenter study of 506 patients. J Clin Oncol 2012;30:abstr 3590.
- Mulcahy MF, Lewandowski RJ, Ibrahim SM, et al. Radioembolization of colorectal hepatic metastases using yttrium-90 microspheres. Cancer 2009;115:1849-58. [PubMed]
- Ricke J, Mohnike K, Pech M, et al. Local response and impact on survival after local ablation of liver metastases from colorectal carcinoma by computed tomography-guided high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys 2010;78:479-85. [PubMed]
- Gray B, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol 2001;12:1711-20. [PubMed]
- Sharma RA, Van Hazel GA, Morgan B, et al. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol 2007;25:1099-106. [PubMed]
- Sharma RA, Wasan HS, Love SB, et al. FOXFIRE: a phase III clinical trial of chemo-radio-embolisation as first-line treatment of liver metastases in patients with colorectal cancer. Clin Oncol (R Coll Radiol) 2008;20:261-3. [PubMed]
- van Hazel GA, Pavlakis N, Goldstein D, et al. Treatment of fluorouracil-refractory patients with liver metastases from colorectal cancer by using yttrium-90 resin microspheres plus concomitant systemic irinotecan chemotherapy. J Clin Oncol 2009;27:4089-95. [PubMed]
- Lim L, Gibbs P, Yip D, et al. A prospective evaluation of treatment with Selective Internal Radiation Therapy (SIR-spheres) in patients with unresectable liver metastases from colorectal cancer previously treated with 5-FU based chemotherapy. BMC Cancer 2005;5:132. [PubMed]
- Hendlisz A, Van den Eynde M, Peeters M, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol 2010;28:3687-94. [PubMed]
- Cosimelli M, Golfieri R, Cagol PP, et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer 2010;103:324-31. [PubMed]
- Kennedy AS, Ball D, Cohen SJ, et al. Safety and efficacy of resin 90y-microspheres in 548 patients with colorectal liver metastases progressing on systemic chemotherapy. J Clin Oncol 2012;30:abstr 264.
- Kennedy A, Ball D, Cohen S, et al. Safety and efficacy of 90Y resin microspheres in elderly (≥70 years) compared to younger patients with colorectal liver metastases (mCRC). J Clin Oncol 2013;31:abstr e14545.
- Kennedy AS. Radiation oncology approaches in liver malignancies. Am Soc Clin Oncol Educ Book 2014;34:e150-5. [PubMed]
- Kennedy AS, Coldwell D, Nutting C, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys 2006;65:412-25. [PubMed]
- Bester L, Meteling B, Pocock N, et al. Radioembolization versus standard care of hepatic metastases: comparative retrospective cohort study of survival outcomes and adverse events in salvage patients. J Vasc Interv Radiol 2012;23:96-105. [PubMed]
- Seidensticker R, Denecke T, Kraus P, et al. Matched-pair comparison of radioembolization plus best supportive care versus best supportive care alone for chemotherapy refractory liver-dominant colorectal metastases. Cardiovasc Intervent Radiol 2012;35:1066-73. [PubMed]
- Jakobs TF, Hoffmann RT, Dehm K, et al. Hepatic yttrium-90 radioembolization of chemotherapy-refractory colorectal cancer liver metastases. J Vasc Interv Radiol 2008;19:1187-95. [PubMed]
- Stubbs RS, O’Brien I, Correia MM. Selective internal radiation therapy with 90Y microspheres for colorectal liver metastases: single-centre experience with 100 patients. ANZ J Surg 2006;76:696-703. [PubMed]
- Hilgard P, Hamami M, Fouly AE, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 2010;52:1741-9. [PubMed]
- Iñarrairaegui M, Thurston KG, Bilbao JI, et al. Radioembolization with use of yttrium-90 resin microspheres in patients with hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol 2010;21:1205-12. [PubMed]
- Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology 2013;57:1826-37. [PubMed]
- Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010;138:52-64. [PubMed]
- Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 2011;54:868-78. [PubMed]
- Raoul JL, Sangro B, Forner A, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev 2011;37:212-20. [PubMed]
- D’Avola D, Lnarrairaegui M, Bilbao JI, et al. A retrospective comparative analysis of the effect of Y90-radioembolization on the survival of patients with unresectable hepatocellular carcinoma. Hepatogastroenterology 2009;56:1683-8. [PubMed]
- Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821-9. [PubMed]
- Bilbao JI, de Martino A, de Luis E, et al. Biocompatibility, inflammatory response, and recannalization characteristics of nonradioactive resin microspheres: histological findings. Cardiovasc Intervent Radiol 2009;32:727-36. [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [PubMed]
- Iñarrairaegui M, Martinez-Cuesta A, Rodríguez M, et al. Analysis of prognostic factors after yttrium-90 radioembolization of advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2010;77:1441-8. [PubMed]
- Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 2008;47:71-81. [PubMed]
- Salem R, Mazzaferro V, Sangro B. Yttrium 90 radioembolization for the treatment of hepatocellular carcinoma: biological lessons, current challenges, and clinical perspectives. Hepatology 2013;58:2188-97. [PubMed]
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011;140:497-507.e2.
- Heckman JT, Devera MB, Marsh JW, et al. Bridging locoregional therapy for hepatocellular carcinoma prior to liver transplantation. Ann Surg Oncol 2008;15:3169-77. [PubMed]
- Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant 2009;9:1920-8. [PubMed]
- Gaba RC, Lewandowski RJ, Kulik LM, et al. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol 2009;16:1587-96. [PubMed]
- Iñarrairaegui M, Pardo F, Bilbao JI, et al. Response to radioembolization with yttrium-90 resin microspheres may allow surgical treatment with curative intent and prolonged survival in previously unresectable hepatocellular carcinoma. Eur J Surg Oncol 2012;38:594-601. [PubMed]
- Leung TW, Lau WY, Ho SK, et al. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys 1995;33:919-24. [PubMed]
- Carr BI. Hepatic arterial 90Yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transpl 2004;10:S107-10. [PubMed]
- Carretero C, Munoz-Navas M, Betes M, et al. Gastroduodenal injury after radioembolization of hepatic tumors. Am J Gastroenterol 2007;102:1216-20. [PubMed]
- Geschwind JF, Salem R, Carr BI, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology 2004;127:S194-205. [PubMed]
- Salem R, Lewandowski RJ, Atassi B, et al. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol 2005;16:1627-39. [PubMed]
- Woodall CE, Scoggins CR, Ellis SF, et al. Is selective internal radioembolization safe and effective for patients with inoperable hepatocellular carcinoma and venous thrombosis? J Am Coll Surg 2009;208:375-82. [PubMed]
- Gil-Alzugaray B, Chopitea A, Inarrairaegui M, et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology 2013;57:1078-87. [PubMed]
- Kennedy AS, Dezarn WA, McNeillie P, et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol 2008;31:271-9. [PubMed]
- King J, Quinn R, Glenn DM, et al. Radioembolization with selective internal radiation microspheres for neuroendocrine liver metastases. Cancer 2008;113:921-9. [PubMed]
- Rhee TK, Lewandowski RJ, Liu DM, et al. 90Y Radioembolization for metastatic neuroendocrine liver tumors: preliminary results from a multi-institutional experience. Ann Surg 2008;247:1029-35. [PubMed]
- Cao CQ, Yan TD, Bester L, et al. Radioembolization with yttrium microspheres for neuroendocrine tumour liver metastases. Br J Surg 2010;97:537-43. [PubMed]
- Saxena A, Chua TC, Bester L, et al. Factors predicting response and survival after yttrium-90 radioembolization of unresectable neuroendocrine tumor liver metastases: a critical appraisal of 48 cases. Ann Surg 2010;251:910-6. [PubMed]
- Memon K, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for neuroendocrine liver metastases: safety, imaging, and long-term outcomes. Int J Radiat Oncol Biol Phys 2012;83:887-94. [PubMed]
- Paprottka PM, Hoffmann RT, Haug A, et al. Radioembolization of symptomatic, unresectable neuroendocrine hepatic metastases using yttrium-90 microspheres. Cardiovasc Intervent Radiol 2012;35:334-42. [PubMed]
- Yang TX, Chua TC, Morris DL. Radioembolization and chemoembolization for unresectable neuroendocrine liver metastases - a systematic review. Surg Oncol 2012;21:299-308. [PubMed]
- Pavel M, Baudin E, Couvelard A, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012;95:157-76. [PubMed]
- Frilling A, Modlin IM, Kidd M, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol 2014;15:e8-e21. [PubMed]
- Kennedy A, Bester L, Salem R, et al. Role of hepatic intra-arterial therapies in metastatic neuroendocrine (net) carcinomas: Guidelines from NET-Liver-Metastases Consensus Conference. HPB: The official journal of the International Hepato Pancreato Biliary Association 2014. [Epub ahead of print].


