The tolerance of gastrointestinal organs to stereotactic body radiation therapy: what do we know so far?
Introduction
The role of stereotactic body radiation therapy (SBRT) in treating gastrointestinal (GI) malignancies is growing in the years since SBRT has become one of the standard therapies for medically inoperable early stage lung cancer patients (1,2). Emami et al. (3) and QUANTEC (quantitative analysis of normal tissue effects in the clinic) (4) described the dose tolerances for conventionally fractionated radiation therapy (RT) with QUANTEC also including dose tolerances of some organs-at-risk (OARs) for hypofractionated schedules. The dose constraints of various abdominal OARs are not very well established (5); Marks et al. comments on using caution in using the linear quadratic (LQ) model in describing dose toxicities for SBRT (4). Currently, there is a wide variation in fractionation schedules, contouring of OARs, and methodologies describing toxicities in SBRT involving GI organs at risk. However, there is emerging consensus that is developing on dose tolerances to GI organs that will be described below.
Liver tolerance in SBRT
Radiation induced liver disease (RILD) is one of the biggest concerns with RT to the liver. As an organ in parallel, the risk of RILD is more dependent on the critical volume irradiated to a dose below the threshold than dose alone. Toxicity to parallel functioning tissues could be reduced by limiting the fractional volume of an organ exposed to a threshold dose (6). The Lyman normal tissue complication probability (NTCP) has been used to describe the volume dependence of RT normal tissue toxicity (7). The model uses three parameters: TD50 (3), the whole liver uniform dose associated with a 50% probability of toxicity, “m”, characterizing the steepness of the dose response at TD50, and “n”, a volume effect parameter that indicates a larger volume effect as it increases ranging from zero to one. The Lyman NTCP model assumes a sigmoid relationship between dose of uniform radiation given to a volume of an organ and the chance of a complication occurring. Dawson et al. used the Lyman NTCP model to evaluate 203 patients treated with conformal liver RT and concurrent hepatic arterial chemotherapy and found that the “n” parameter was larger than previously described, suggesting a strong volume effect for RILD and a correlation of NTCP with mean liver dose (8). In addition, she found that the liver had a low threshold volume for RILD and that the RT tolerance of the liver was reduced in patients with primary liver cancer compared to metastasis to the liver (9). The threshold mean liver dose to develop RILD was determined to be 30 Gy above which there was approximately 4% per Gy increase in risk. The mean liver dose associated with a 5% risk of RILD for patients with metastatic liver disease was 37 Gy in 1.5 Gy/fraction and 32 Gy in 2 Gy/fraction versus 32 Gy in 1.5 Gy/fraction and 28 Gy in 2 Gy/fraction for patients with primary liver disease. Based on these results among others, a phase I/II study was developed to give highly individualized SBRT treatment to patients with primary liver cancer based on effective liver volume irradiated (Veff) (10). When the Veff <25%, doses of 54 Gy (9 Gy ×6 fractions) were delivered safely to hepatocellular carcinoma (HCC) lesions with good local control. As the Veff increased the dose delivered safely decreased. Long-term outcomes demonstrated that first site of recurrence was in the unirradiated volume of the liver suggesting that combining regional or systemic therapies with SBRT may lead to improved local control. This is currently a question being addressed in the ongoing RTOG 1112 study of SBRT versus SBRT followed by sorafenib for patients with localized Child-Turcotte-Pugh’s class (CTP) A.
For patients with HCC, the group at the Indiana University performed a phase I dose escalation trial to determine the feasibility and toxicity of treatment (11). Patients were eligible for the study if they were CTP A or B, not candidates for resection, had one to three lesions and cumulative tumor diameter less than or equal to 6 cm. Dose escalation started at 36 Gy in three fractions with a subsequent planned escalation of 2 Gy/fraction/ level. Dose limiting toxicity (DLT) was defined by the Common Terminology Criteria for Adverse Events (CTCAE) v3.0. DLT was reached in CTP-B patients when two patients on trial developed grade 3 hepatic toxicity at the 42 Gy at 14 Gy/fraction level with one patient experiencing progressive liver failure. As a result of the observed toxicities, CTP-B patients were then treated with a dose of 40 Gy in 5 fractions (8 Gy per fraction). The only factor related to more than a grade 3 toxicity or death within six months of treatment was the CTP score which was safe for CTP-A and CTP-B with score of 7. In a follow-up study the same group showed that in a series of sixty patients treated with liver confined HCC, only 12% of patients with a CTP score ≤7 experienced an increase of greater than one grade in hematologic/hepatic dysfunction (12). In another follow-up analysis of phase I and II trial for HCC, Bujold et al. (13) describes 102 patients with CTP class A who were treated to 24 to 54 Gy in 6 fractions. Although the treatment was generally well tolerated, there was a significantly higher mean liver dose for those who developed grade 5 toxicity (18.1 vs. 15.4 Gy, P=0.02).
For patients with liver metastasis there has been several Phase I and II studies looking at various fractionation schemes ranging from 26 Gy ×1 (14) to 20 Gy ×3 (15) with actuarial local control of 67% at eighteen months and 92% at two years respectively. Inclusion criteria for the 20 Gy ×3 fraction study included tumor diameter less than 6 cm, no prior RT to the upper abdomen, total bilirubin less than 3 mg/dL, albumin greater than 2.5 g/dL, normal prothrombin/partial thromboplastin times unless on anticoagulants, serum liver enzymes less than three times the upper limit of normal, no ascites on exam, and normal renal function. There was only one instance of CTCAE v3.0 grade 3 toxicity to soft tissue observed in a cohort of 47 patients with 63 lesions treated; with no grade 4 or 5 toxicity. The actuarial rate of any grade ≥3 toxicity was 2% at last follow up with a median of 16 months. QUANTEC has provided dose limits that are noted in Table 1 in addition to dose, fractionation schedule and toxicities reported in a select group of liver SBRT studies. Based on the low rates of RILD observed in multiple phase I and II trials of SBRT for liver metastasis, it appears that if one adheres to the liver constraints used in those studies, the anticipated incidence of RILD should be low.
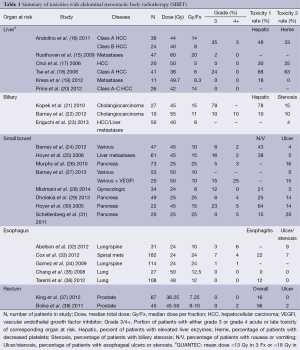
Full table
Patients with HCC deserve separate consideration. For CTP-A patients, the use of a risk-adapted approach as used in the University of Toronto trial (13) or the dose constraints used for CTP-A patients in the Indiana University phase I trial and the subsequent retrospective study (11,12) appeared to yield a low incidence of RILD. For CTP-B patients, a 5-fraction regimen is recommended (11,12); the dose to one-third of the uninvolved liver should be restricted to ≤18 Gy (3.6 Gy/fraction), and ≥500 cc of uninvolved liver should receive <12 Gy (2.4 Gy/fraction). For patients with a CTP score of ≥8, the risk of RILD will be much higher and SBRT may not be safe unless they are already listed from liver transplantation (11,12).
Biliary tract injury in SBRT
Biliary tract cancers have a dismal prognosis if patients are not surgical candidates (39,40). At Thomas Jefferson University Hospital, patients received RT as part of a combined modality approach with external beam RT for an average of 46 Gy for five days per week, brachytherapy implants using Ir-192 sources with a mean dose of 25 Gy at 1 cm and chemotherapy of either 5-fluorouracil (5FU) alone or combined with Adriamycin or mitomycin-C (41). A dose response was shown for patients who received greater than 55 Gy up to 60-75 Gy with improved median survival as compared to surgical resection or chemotherapy, neither producing statistically significant benefits as independent variables. These results among others led to a prospective study that used SBRT to a total dose of 45 Gy in three fractions given in five to eight days for patients with unresectable cholangiocarcinoma in Denmark (21). Twenty-six of the twenty-seven patients on study had Klatskin tumors with the other patient having intrahepatic cholangiocarcinoma. Patients tolerated treatment well acutely up to two months after treatment with progression free survival and overall survival similar to external beam studies. Eight of the 27 patients suffered grade 3 hyperbilirubinemia, but no other biliary toxicity was noted.
Barney et al. (24) performed a single-institutional study of ten patients treated for unresectable primary or recurrent cholangiocarcinoma with SBRT. Patients were treated to a median prescription dose of 55 Gy (range, 45-60 Gy) in three or five daily consecutive fractions over a week. Eight patients were treated with the 5-fraction regimen while the remaining were treated with 3 fractions. Dose constraints were used for organs at risk including bowel structures of stomach, duodenum and intestine with a maximum point dose of 32 Gy and a 10 cc constraint of 20 Gy. For the liver, at least 700 cc of normal liver was constrained to receive less than 21 Gy. Treatment response and toxicities were graded over a median of fourteen months. Local control within the SBRT treatment field was 100% with four patients having progression elsewhere in the liver. Toxicity was graded by CTCAE v4.0 with the most common early toxicities of nausea, vomiting and GI pain with no acute grade ≥3 toxicity. Late ≥2 toxicities included grade 2 GI pain, one patient with grade 3 biliary stenosis requiring stenting and one patient with grade 5 liver failure. The patient who developed grade 3 biliary stenosis did not have a constraint placed on the biliary tract itself with portions in the high-dose volume. The patient who developed liver failure was treated 60 Gy in 5 fractions. The plan met part of the QUANTEC constraint of >700 cc of normal liver to receive ≤15 Gy (Dmax <15) with their recommendation for 3 fraction SBRT to keep mean liver dose <13 or <18 Gy in 6 fraction SBRT. The patient’s mean liver dose was 19.3 Gy in 5 fractions with a liver volume of 1980.4 and 1,051.4 cc receiving <15 Gy. This patient had significant prior chemotherapy for breast cancer and autologous bone marrow transplant which the authors speculate may have caused subclinical liver injury that may have led with SBRT to liver failure.
A detailed dosimetric evaluation of central biliary system (CBS) toxicity after SBRT has recently been reported by Eriguchi et al. (23). To date, this is the first description of standardized contouring of the CBS and evaluation of patients who were irradiated to >20 Gy to the CBS. Fifty patients were treated to lesions in the liver with doses depending on lesion type of 40 Gy for CTP-A patients with HCC, 35 Gy for CTP-B with HCC and 50 Gy for liver metastasis all in 5 fractions. The median follow-up period was 18.2 months with toxicity graded using CTCAE v4.0. Dose volume histogram (DVH) data was converted to dose length histograms (DLH) to get detailed information on biliary toxicity. There were two cases of grade 1 bile duct stenosis that presented with bile duct dilatation downstream of the stenosis. One patient had two treatments of TACE and SBRT (each 40 Gy in 5 fractions). Stricture was noted one year after the second SBRT treatment corresponding to a site irradiated to a cumulative maximum dose of 88 Gy. In this patient >6 cm of the entire biliary tract was irradiated to >50 Gy and almost 1.3 cm of the left hepatic duct was irradiated to >80 Gy. The patient was treated symptomatically and was reported alive without recurrence 52 months after the first SBRT. The second patient’s biliary stricture did not correlate to the >20 Gy region of biliary tract irradiated and only 7 mm of the biliary tract received >20 Gy with 0 mm receiving >30 Gy. None of the patients in the study developed obstructive jaundice or biliary infection. When evaluating DLH data for the whole cohort, thirteen patients (26%) were irradiated between 40 and 55 Gy to >1 cm of the CBS with no biliary toxicity, and seven patients (14%) were irradiated with >20 Gy to >20% of the gallbladder with no gallbladder toxicity observed. The authors conclude that 40 Gy in 5 fractions is safe with minimal biliary toxicity. There is heterogeneity in the dose regimens and toxicities described when treating lesions in the CBS as described in the studies above. Of note, biliary tract injury is a key toxicity in treating with SBRT that is frequently overlooked.
Gastric, duodenal and small bowel toxicities in SBRT
Gastric, duodenal and small bowel toxicities are a significant consideration in SBRT planning for GI malignancies. There has been an evolution of data that is currently used to guide dose constraints for these organs. In a case report, Furman et al. (42) described a patient treated with SBRT for a liver metastasis at 50 Gy in 10 fractions, who consequently suffered a gastric perforation presumably because there was too much dose to the gastric wall. Hoyer et al. (30) conducted a phase II study of twenty-two patients with locally advanced unresectable pancreatic cancer who were treated with 45 Gy in 3 fractions within 5-10 days. All tumors in the study were no more than 6 cm in largest diameter. Treatment planning involved an expansion from GTV to CTV to include peri-tumoral edema with a PTV margin of 5 mm transversally and 10 mm cranio-caudally. Only two patients (9%) were found to have a partial response, median survival time was 5.7 months and only 5% were alive one year after treatment. The group reported a significant rate of toxicity fourteen days after treatment graded 2 or higher in 79% of patients. Four of the twenty-two patients (18%) experienced severe mucositis or ulceration of the stomach or duodenum and one had a nonfatal perforation requiring surgery. The authors reported that the median volume receiving ≥30 Gy was 136 cc (range, 38-376 cc).
In a following Phase II study of SBRT for colorectal metastasis by the same group, sixty-four patients with a total number of 141 lesions in the abdomen from various malignancies was treated with the same fractionation of 45 Gy in 3 fractions within five to eight days (25). The dose to the intestine and stomach was restricted to as low as possible. Toxicity was evaluated in sixty-one patients. Progression of toxicity to grade 2 or higher or performance status 2 or higher was observed in 48% of patients within 6 months after SBRT. One death was reported due to hepatic failure. One patient had perforation of a colonic ulceration requiring surgery and two patients with duodenal ulceration were treated conservatively. In all cases at least part of the stomach or duodenum received a total dose of 30 Gy or higher (67% of the prescribed dose).
The previously mentioned Kopek et al. trial also used 45 Gy in 3 fractions, but for cholangiocarcinomas. Gastroduodenal ulceration was seen at a median of 6.7 months after treatment in six of the twenty-seven patients (22%) requiring hospitalization and transfusion; while four patients developed duodenal stenosis (11%) with half requiring dilatation. This rate of small bowel injury could be due to the location of tumors treated with all but one tumor in the hilar location with close proximity to duodenum/small bowel. The mean volume of duodenum receiving higher dose was greater in the group with ulceration and stenosis; however, the difference was not statistically significant. A statistically significant association between grade ≥2 ulceration (graded by CTCAE v.3) and volume of duodenum treated to a given dose level could not be determined. The mean maximum dose to 1 cm3 of duodenum (Dmax 1 cc) was significantly higher for patients with grade ≥2 ulceration or stenosis 37.4 Gy (83% of prescription dose) versus 25.3 Gy (56% of prescription dose). Also observed was that a mean duodenal Dmax 1 cc of 25.3 Gy in three fractions (BED 96 Gy, α/β=3) was found in patients with grade 0 or 1 duodenal toxicity. Based on these findings and comparing their data to others published at the time the constraint followed by this group is 1 cc of duodenum to get no more than 21 Gy in three fractions (V21 Gy ≤1 cc) for all abdominal SBRT at the investigators institution. Bae et al. (43) further investigated the potential toxicity threshold when they retrospectively examined patients treated by stereotactic ablative radiotherapy (SABR) at 33-60 Gy in 3 fractions and determined that severe intestinal toxicity was 18% for patients treated in 3 consecutive days compared to 0% in those treated in 4 to 8 days. Their recommended parameters for gastroduodenal dose includes V20 <14 mL, V25 <7 mL, V30 <5 mL, V35 <1 mL and Dmax <45 Gy. The same group also determined that Dmax can be a valuable predictor of gastroduodenal toxicity as the maximum point dose of 35 and 38 Gy in the gastroduodenum were respectively associated with a 5% and 10% probability of developing severe gastroduodenal toxicity (44), and that the best predictor of intestinal toxicity was V25Gy >20 mL. In a similar dosimetric analysis of SABR treatment for gynecologic malignancies, Mislmani et al. (28) concluded that patients whose duodenal volume received 80% or more of the prescribed dose, in this case 24 Gy in 3 fractions, were more likely to suffer GI toxicity.
Prior to these publications, Koong and colleagues performed a single fraction dose escalation study for patients with locally advanced pancreatic cancer (45). The dose was escalated from 15 to 20 or 25 Gy with evaluation of acute GI toxicity scored by the Radiation Therapy Oncology Group (RTOG) criteria and treatment response. The small bowel was constrained by allowing the 50% isodose line to cover only the duodenal wall closest to the tumor. The median treated tumor volume was 29 cc (range, 19.2 to 71.9 cc). The median overall survival time was 11 months, in the six patients at the highest dose level the median survival time was 8 months and all patient had local control at the time of last follow up. There was no significant acute GI toxicity within the three-month follow up period. There was no grade 3 or higher acute GI toxicity observed. Two patients reported grade 1 nausea, two patients reported grade 2 abdominal pain and one patient reported grade 2 diarrhea with all these symptoms resolving. This study provided the dosimetric information of mean dose to 50% and 5% of the duodenum and bowel of 14.5 and 22.5 Gy respectively at the 25 Gy dose level.
These findings were used to create bowel constraints that this group has used in subsequent studies including a study on a dosimetric model of duodenal toxicity for SBRT (26). Seventy-three patients with locally advanced unresectable pancreatic adenocarcinoma were treated with 25 Gy in one fraction of which sixty-three (86%) of patients received gemcitabine based chemotherapy from 2002 through 2007. The median time between the last dose of gemcitabine and SBRT was fourteen days. The target volume was the GTV plus a 2 to 3 mm isotropic expansion for PTV with the median PTV of 43 cm3 (range, 10-96 cm3). The contouring volumes of the duodenum were elegantly described giving a quantitative basis for reported toxicity. The superior extent of the duodenum was 1.0 cm beyond the superior extent of the PTV, not to extend past the midpoint of the pylorus. The inferior extent was contoured 1.0 cm beyond the inferior extent of the PTV. The duodenum volume was only contoured on axial slices where a portion of the duodenum extended within a 3 cm radius surrounding the PTV. The duodenum was constrained by: 5% of the volume received <22.5 Gy (V22.5 <5%), V12.5 <50% and with the 50% isodose line not to reach the distal wall of the duodenal lumen on CT imaging. Toxicity was reported using CTCAE v3.0. DVH endpoints evaluated included V5, V10, V15, V20, V25, Dmax, as well as NTCP. The group found that 12 patients (16%) experienced grade 2-4 duodenal toxicity with a median time to symptoms of 6.3 months (range, 1.6-11.8 months). Using the data from these 12 patients, they found that keeping V15 <9.1 cm3 or V20 <3.3 cm3 reduced the 12 months toxicity rate from 52% to 11%. In addition if Dmax <23 Gy the 12 months toxicity rate decreased from 49% to 12%. Using the NTCP model, they found no toxicity if the NTCP value was <6% with 83% of duodenal toxicity associated with an NTCP value of >15%. This study specified contouring volumes for tumor as well as normal organs at risk and dose constraints in both absolute and relative terms.
Single fraction SBRT has been used with sequential standard dose gemcitabine to evaluate toxicity, local control and overall survivals in twenty patients (31). All patients completed SBRT to a median 40.8 cc (range, 12.1 to 84.3 cc) PTV volume and a median of five cycles of chemotherapy. Acutely there were 3 patients (15%) who experienced grade 2 GI toxicity within 4 to 12 months form SBRT. All ulcers were managed medically and resolved with proton pump inhibitor therapy. Late grade 3 or greater GI toxicity occurred in one patient (5%) who developed a grade 4 duodenal perforation treated with surgery. No patients developed non-hematologic acute grade 3 or greater toxicity. No toxicities were observed more than one year after SBRT. The duodenum dose was prioritized above all other organs at risk during planning using the same duodenal dose limits developed by this group of V22.5 <5% and V12.5 <50%.
In addition to single fraction and three fraction SBRT as options for patients with locally advanced unresectable pancreatic adenocarcinoma as described above, a five fraction regimen has been employed with a duodenal toxicity profile that is relatively well tolerated (22). At Mayo Clinic, forty-seven patients with fifty lesions in close proximity to the stomach, duodenum, small bowel and colon were evaluated for toxicity including three pancreas lesions between May 2008 and February 2010 with the most common prescribed dose 50 Gy in five fractions over five consecutive days. Tumor GTV was equal to CTV with a 5 mm expansion for PTV. Specific constraints for the five fraction 50 Gy treatment was V38 ≤5 cc, V32.5 ≤15 cc, V20 ≤30 cc and Dmax <42 Gy. Toxicity was graded using CTCAE v.3 over a median follow-up of 12 months (range, 2 to 28 months). Kaplan-Meier estimates of local control, overall survival, and freedom from metastasis was reported at both 6 and 12 months of 98%, 90%, 63% and 87%, 62%, 37%, respectively. There were no grade ≥3 acute toxicities reported. Fifteen patients (30%) reported grade 1 nausea and/or vomiting and five patients (10%) reported grade 2 nausea and/or vomiting. There were three reported grade 3 toxicities with two patients having grade 3 biliary stenosis requiring stent placement and one patient developing a grade 3 perforation of the stomach requiring a temporary nasojejunal feeding tube and intra-abdominal drain. There was one grade 5 perforation leading to death in a patient with unresectable acinar cell carcinoma of the pancreas who was taken to surgery for resection of the bowel and exploration and was found to have local tumor invasion that may have contributed to the perforation. In addition this patient had prior RT to a volume that overlapped the SBRT volume to a total dose of 50.4 Gy in 28 fractions two years prior. Both patients who developed GI perforations had prior bevacizumab which is associated with an increased incidence of bowel ulceration and perforation with and without radiotherapy (46). To further evaluate the question of increased risk of serious bowel injury, graded 3-5 using CTCAE v4.0, after bevacizumab and SBRT, Barney et al. looked at all the patients (seventy-six total) treated in their center between May 2008 and August 2011 who received a vascular endothelial growth factor inhibitor (VEGFI) within two years after SBRT (27). Seven patients (9%) had a serious bowel injury at a median of 4.6 months after SBRT. If patients received VEGFI within three months of completing SBRT the rate of serous bowel injury was 38%. This suggests that other treatment factors can synergistically affect rates of GI toxicity after SBRT.
Recently a multi-center Phase II study of forty-nine patients who received sequential gemcitabine followed by five fraction SBRT of 33 Gy delivered in one to two weeks was completed and reported in abstract form and personal communication (29). Dose constraints for proximal duodenum and stomach were V33 ≤1 cc, V20 ≤3 cc, and V15 ≤9 cc with toxicities graded using CTCAE v4.0. Treatment volume was GTV and a 2-3 mm margin for PTV with no CTV expansion. Proximal duodenum, small bowel and stomach were contoured 1 cm above and below the PTV. Median follow-up after SBRT was 9.9 months with median treatment volume of 71.4 cm3 (range, 31.9-225.2 cm3). Acute grade two or higher toxicity rate was 16.3%. Late grade 2 or higher toxicities were reported in 5 patients (11%). These included one case of grade 2 enteritis, three cases of grade 3 ulcer and one case of grade 4 fistula. The toxicity endpoints of the study were met as the toxicity seen was less than 40% late > grade 2 toxicity seen in the single fraction regimen at Stanford (47).
In 2010 QUANTEC provided recommendations for dose constraints on the stomach and small bowel in both absolute and relative terms based on data available at that time (48). The volume of the stomach receiving greater than 22.5 Gy was limited to less than 4% or 5 mL with a maximum point dose of less than 30 Gy for 3 fraction SBRT. The volume of the small bowel receiving greater than 12.5 Gy to less than 30 mL if using single fraction with a maximum point dose of less than 30 Gy for 3 to 5 fraction SBRT. As studies are continually reported with detailed contouring guidelines of normal tissue and organs at risk as well as detailed DVH dose constraints and toxicity outcomes; as outlined in the studies above, we will have improved information to guide our treatments.
Esophageal toxicities in SBRT
Dose to the esophagus is a concern for patients undergoing SBRT for lesions in the thoracic cavity or spine. At Stanford University a retrospective analysis was conducted on thirty-one patients who had lesions less than 1 cm in the axial plane from the thoracic esophagus treated between December 2004 and November 2009 (32). GTV was equal to CTV and expansions to PTV were up to 2 mm for spine lesions and 5 mm for lung lesions. The esophagus was contoured 3cm above and below the extent of the PTV. Patients were treated using either multiple fractionation regimens between 16-50 Gy over one to five fractions whereas patients treated with single fraction received doses ranging from 16-24 Gy. In order to correlate effects of single fractionation treatment versus multi-fractionated treatment the LQ model was used to determine a biologically effective dose (BED) for multi-fractionated treatment into a single fraction biologically effective dose (SFBED) using α/β=3. This SFBED was converted using the linear quadratic-linear (LQ-L) model to account for the possible inaccuracy of the LQ model in treatments with fraction size >8 Gy (49). Dose constraint to the esophagus used initially for single fraction treatment was a maximum point dose of 20 Gy with 50% of the esophagus restricted to <10 Gy with a later constraint of V12-14 Gy <2-5 cm3. Esophageal toxicities were graded using CTCAE v4.0. Estimated rates of greater than a grade 2 esophageal toxicity was 7% at six months and 14% at twelve months. There were three esophageal toxicities reported including grade 2 esophagitis, grade 4-5 tracheoesophageal fistula and grade 4-5 esophageal perforation with both grade 4-5 toxicities leading to death. The median time to develop esophageal toxicity was 4.1 months (range, 0.6-6.1 months). Both patients who developed grade 4-5 toxicities had exposure to chemotherapy as part of their treatment course. The location of toxicity was within the high-dose radiation volume. The grade 5 adverse events occurred at SFBED to D5 cc (minimum dose in Gy to 5 cm3 of esophagus receiving the highest dose) of 16.5 and 11.4 Gy, to D2 cc of 18.2 and 14.1 Gy, and to Dmax (maximum dose to 0.01 cm3) of 21.0 and 18.5 Gy by LQ modeling. Corresponding values by LQ-L model were 16.5 and 13.2 Gy, 18.2 and 18.2 Gy, and 21.0 and 27.3 Gy, respectively. Prior reported safe thresholds doses to the esophagus for single fraction treatment were D5 cc of 14.5 Gy, D2 cc of 15-20 Gy, Dmax of 19 Gy (34,50) were compared to the values achieved in this study and found to be lower than the published thresholds in some cases. Based on these findings, the dose limit for the esophagus following single fraction SBRT is Dmax of 15.4 Gy and D5 cc of 11.9 Gy.
More recently the Memorial Sloan-Kettering group looked at esophageal toxicity, graded by CTCAE v4.0, after single fraction SBRT for spinal metastasis abutting the esophagus in 182 patients treated between 2003 and 2010 (33). The volume of disease included GTV and CTV of any abnormal marrow signal suspicious for microscopic involvement and a margin of normal bone to account for subclinical spread. PTV was created by expanding CTV by ≥2 mm. The esophagus was defined as a solid structure including all layers of the esophageal wall and luminal contents extending 2 cm superior and inferior to the PTV. Initially the dose constraint used for the esophagus was at the discretion of the treating physician and was subsequently instituted as ≤15 Gy to 2 cm3 of esophagus with an allowable deviation of 20 Gy to 2 cm3 of esophagus at the discretion of the treating physician in 2009 (34). In April 2010, the permitted constraints included ≤20 Gy to 2 cm3 and ≤14 Gy to 4 cm3. The median treatment dose was 24 Gy (range of 16-24 Gy) with a median follow-up of 12 months (range, 3-81 months). Acutely there were thirty-one patients (15%) with toxicities reported including twenty-eight patients with grade 1 or 2 toxicities, one patient with grade 3 toxicity and two patients with grade 4 toxicities. There were no acute grade 5 toxicities reported. There were twenty-four patients with late toxicities (≥90 days after treatment): 13 (6%) grade 1 or 2, six (3%) grade 3, four (2%) grade 4 and one (≤1%) grade 5. The eleven grade ≥3 toxicities included: esophageal stenosis (5 patients), tracheoesophageal fistula (4 patients) and esophageal ulcer (2 patients). All patients who developed ≥4 toxicity had prior chemotherapy, iatrogenic manipulation of the esophagus or both. An atlas of complication incidence was created using absolute volume DVHs from all treatment plans in the cohort. The atlas demonstrates that the probability of grade >3 toxicity is a function of dose and volume of irradiated esophagus. The model suggests that if the dose to the hottest 2.5 cm3 of esophagus <14 Gy yields a grade >3 toxicity rate of <5% with a steep increase in toxicity after further increases in dose. In addition there is a 10% risk of grade ≥3 if the dose to this volume is increased to 18 Gy and a 15% if 20 Gy is delivered. Based on these findings the group’s esophageal constraints is 14 Gy to 2.5 cm3, V12 <3.78 cm3, V15 <1.87 cm3 and V20 <1.87 cm3 with a maximum point dose of to the esophagus of <22 Gy.
Rectal toxicities in SBRT
Treating prostate cancer with SBRT has yielded toxicity data that provides an intrinsically different normal tissue and tumor radiobiology. Two large retrospective series demonstrated that SBRT was tolerated very well by prostate cancer patients. Friedland described 112 low and intermediate risk prostate cancer patients who were treated to 35 Gy in 5 fractions and suffered no high grade gastrointestinal toxicity (51). At a slightly elevated dose of 36.25 Gy in five fractions, 254 patients with localized prostate cancer also endured treatment without any notable rectal toxicity (52). Prospectively, between 2003 and 2009 there were 67 patients treated with SBRT to a total of 36.25 Gy in five fractions to PTV which consisted of a volumetric expansion of GTV to PTV with a volumetric expansion of the prostate by 5 mm except 3 mm posteriorly (37). The DVH goals from the rectum was V50% <50%, V80% <20%, V90% <10%. Patients’ assessments were evaluated using the RTOG rectal toxicity scale. Median follow up was 2.7 years (range, 1.8 to 4.5 years). Rectal grade 3, 2 and 1 toxicities were seen in 0, 2% (1 patient) and 12.5% (37). Persistent rectal bleeding was not observed. Fractionation schedule made a difference in that every other day (QOD) treatment showed fewer low grade toxicities compared to every day treatment. There was a seven fold reduction in grade 1 rectal toxicity in favor of QOD treatment. A similar trial by Masden et al. (53) described 40 early stage prostate cancer patients who received 33.5 Gy in five fractions, none of whom suffered any grade 3 rectal toxicity. In a phase I dose escalation trial, Boike et al. (38) analyzed the toxicities of treating low and intermediate risk prostate cancer with either 45, 47.5, or 50 Gy in 5 fractions. The anterior, lateral, and posterior rectal walls were limited to 105%, 90%, and 45% of the prescribed dose, respectively. Low grade (≤2) rectal toxicity was relatively similar for each group at 47-67%. There was one patient in the 50-Gy group with a grade 4 rectal ulcer, who was on immunosuppressant for a kidney transplant.
Conclusions
Recent SBRT trials indicate that stereotactic body radiotherapy can be delivered to abdominal and thoracic tumors with a toxicity profile similar to that of standard fractionation. SBRT should be used with caution for patients with a poor baseline performance status and those with prior or future systemic therapy, especially VEGFI, as these were the patients who disproportionately suffered grade 3 and 4 toxicities. Review of the literature indicates that in SBRT treatment involving GI structures, standardization in contouring organs at risk, fractionation schedule, length of treatment, and volume of organ exposed to a particular dose affects the rate of short and long term toxicity. While recent studies shed some light on dose optimization for SBRT, there is a definite need for further trials or institutional cooperation to combine multiple databases to yield enough robust data to make treatment planning guidelines that can uniformly lead to reductions in toxicity to GI structures. As more data is collected and reported, the guidelines for dose to critical GI organs will be refined to maximize treatment benefit and minimize toxicity.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [PubMed]
- Lo SS, Fakiris AJ, Chang EL, et al. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol 2010;7:44-54. [PubMed]
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109-22. [PubMed]
- Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010;76:S10-9. [PubMed]
- Lo SS, Sahgal A, Chang EL, et al. Serious complications associated with stereotactic ablative radiotherapy and strategies to mitigate the risk. Clin Oncol (R Coll Radiol) 2013;25:378-87. [PubMed]
- Hall EJ. Do no harm--normal tissue effects. Acta Oncologica 2001;40:913-6. [PubMed]
- Lyman JT. Normal tissue complication probabilities: variable dose per fraction. Int J Radiat Oncol Biol Phys 1992;22:247-50. [PubMed]
- Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 2002;53:810-21. [PubMed]
- Dawson LA, Ten Haken RK. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol 2005;15:279-83. [PubMed]
- Dawson LA, Eccles C, Craig T. Individualized image guided iso-NTCP based liver cancer SBRT. Acta Oncologica 2006;45:856-64. [PubMed]
- Cárdenes HR, Price TR, Perkins SM, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol 2010;12:218-25. [PubMed]
- Andolino DL, Johnson CS, Maluccio M, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2011;81:e447-53. [PubMed]
- Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 2013;31:1631-9. [PubMed]
- Herfarth KK, Debus J, Lohr F, et al. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol 2001;19:164-70. [PubMed]
- Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009;27:1572-8. [PubMed]
- Andolino DL, Forquer JA, Henderson MA, et al. Chest wall toxicity after stereotactic body radiotherapy for malignant lesions of the lung and liver. Int J Radiat Oncol Biol Phys 2011;80:692-7. [PubMed]
- Choi BO, Jang HS, Kang KM, et al. Fractionated stereotactic radiotherapy in patients with primary hepatocellular carcinoma. Jpn J Clin Oncol 2006;36:154-8. [PubMed]
- Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2008;26:657-64. [PubMed]
- Kress MS, Collins BT, Collins SP, et al. Stereotactic body radiation therapy for liver metastases from colorectal cancer: analysis of safety, feasibility, and early outcomes. Front Oncol 2012;2:8. [PubMed]
- Price TR, Perkins SM, Sandrasegaran K, et al. Evaluation of response after stereotactic body radiotherapy for hepatocellular carcinoma. Cancer 2012;118:3191-8. [PubMed]
- Kopek N, Holt MI, Hansen AT, Hoyer M. Stereotactic body radiotherapy for unresectable cholangiocarcinoma. Radiother Oncol 2010;94:47-52. [PubMed]
- Barney BM, Olivier KR, Miller RC, et al. Clinical outcomes and toxicity using stereotactic body radiotherapy (SBRT) for advanced cholangiocarcinoma. Radiat Oncol 2012;7:67. [PubMed]
- Eriguchi T, Takeda A, Sanuki N, et al. Acceptable toxicity after stereotactic body radiation therapy for liver tumors adjacent to the central biliary system. Int J Radiat Oncol Biol Phys 2013;85:1006-11. [PubMed]
- Barney BM, Olivier KR, Macdonald OK, et al. Clinical outcomes and dosimetric considerations using stereotactic body radiotherapy for abdominopelvic tumors. Am J Clin Oncol 2012;35:537-42. [PubMed]
- Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncologica 2006;45:823-30. [PubMed]
- Murphy JD, Christman-Skieller C, Kim J, et al. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys 2010;78:1420-6. [PubMed]
- Barney BM, Markovic SN, Laack NN, et al. Increased bowel toxicity in patients treated with a vascular endothelial growth factor inhibitor (VEGFI) after stereotactic body radiation therapy (SBRT). Int J Radiat Oncol Biol Phys 2013;87:73-80. [PubMed]
- Mislmani M, Frasure H, Suppiah S, et al. Acute gastrointestinal toxicity after robotic stereotactic ablative radiotherapy for treatment of metastatic gynecological malignancies. Future Oncol 2014;10:241-8. [PubMed]
- Dholakia AS, Chang DT, Goodman KA, et al. A Phase 2 Multicenter Study to Evaluate Gemcitabine and Fractionated Stereotactic Body Radiation Therapy for Locally Advanced Pancreatic Adenocarcinoma. Int J Radiat Oncol Biol Phys 2013;87:S28.
- Hoyer M, Roed H, Sengelov L, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol 2005;76:48-53. [PubMed]
- Schellenberg D, Kim J, Christman-Skieller C, et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2011;81:181-8. [PubMed]
- Abelson JA, Murphy JD, Loo BW Jr, et al. Esophageal tolerance to high-dose stereotactic ablative radiotherapy. Dis Esophagus 2012;25:623-9. [PubMed]
- Cox BW, Jackson A, Hunt M, et al. Esophageal toxicity from high-dose, single-fraction paraspinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2012;83:e661-7. [PubMed]
- Gomez DR, Hunt MA, Jackson A, et al. Low rate of thoracic toxicity in palliative paraspinal single-fraction stereotactic body radiation therapy. Radiother Oncol 2009;93:414-8. [PubMed]
- Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;72:967-71. [PubMed]
- Taremi M, Hope A, Dahele M, et al. Stereotactic body radiotherapy for medically inoperable lung cancer: prospective, single-center study of 108 consecutive patients. Int J Radiat Oncol Biol Phys 2012;82:967-73. [PubMed]
- King CR, Brooks JD, Gill H, et al. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:877-82. [PubMed]
- Boike TP, Lotan Y, Cho LC, et al. Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol 2011;29:2020-6. [PubMed]
- Farley DR, Weaver AL, Nagorney DM. “Natural history” of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin Proc 1995;70:425-9. [PubMed]
- Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 2001;234:507-17; discussion 517-9. [PubMed]
- Alden ME, Mohiuddin M. The impact of radiation dose in combined external beam and intraluminal Ir-192 brachytherapy for bile duct cancer. Int J Radiat Oncol Biol Phys 1994;28:945-51. [PubMed]
- Furman MJ, Whalen GF, Shah SA, et al. Gastric perforation following stereotactic body radiation therapy of hepatic metastasis from colon cancer. Pract Radiat Oncol 2013;3:40-4. [PubMed]
- Bae SH, Kim MS, Kim SY, et al. Severe intestinal toxicity after stereotactic ablative radiotherapy for abdominopelvic malignancies. Int J Colorectal Dis 2013;28:1707-13. [PubMed]
- Bae SH, Kim MS, Cho CK, et al. Predictor of severe gastroduodenal toxicity after stereotactic body radiotherapy for abdominopelvic malignancies. Int J Radiat Oncol Biol Phys 2012;84:e469-74. [PubMed]
- Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2004;58:1017-21. [PubMed]
- Badgwell BD, Camp ER, Feig B, et al. Management of bevacizumab-associated bowel perforation: a case series and review of the literature. Ann Oncol 2008;19:577-82. [PubMed]
- Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2008;72:678-86. [PubMed]
- Kavanagh BD, Pan CC, Dawson LA, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys 2010;76:S101-7. [PubMed]
- Park C, Papiez L, Zhang S, et al. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:847-52. [PubMed]
- Timmerman RD. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol 2008;18:215-22. [PubMed]
- Friedland JL, Freeman DE, Masterson-McGary ME, et al. Stereotactic body radiotherapy: an emerging treatment approach for localized prostate cancer. Technol Cancer Res Treat 2009;8:387-92. [PubMed]
- Katz AJ, Santoro M, Ashley R, et al. Stereotactic body radiotherapy for organ-confined prostate cancer. BMC Urol 2010;10:1. [PubMed]
- Madsen BL, Hsi RA, Pham HT, et al. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized disease: first clinical trial results. Int J Radiat Oncol Biol Phys 2007;67:1099-105. [PubMed]


