Should we move beyond VEGF inhibition in metastatic colorectal cancer? Lessons from early phase clinical trials
Introduction
Agents targeting the angiogenic pathway have been the cornerstone of metastatic colorectal cancer (mCRC) treatment in recent years. Standard therapy includes systemic chemotherapy, in combination or in sequence, consisting of fluoropyrimidines, oxaliplatin, and irinotecan with monoclonal antibodies that target vascular endothelial growth factor (VEGF), bevacizumab or ziv-aflibercept (1).
The benefit of adding bevacizumab was demonstrated in the AVF2017 phase III study of previously untreated patients randomized to irinotecan plus bolus fluorouracil and leucovorin (IFL) with placebo or bevacizumab (2). In 2004, the N9741 study reported that IFL was an inferior backbone compared to fluorouracil, folinic acid, and oxaliplatin (FOLFOX) (3). With subsequent studies showing equal efficacy of FOLFOX or FOLFIRI based chemotherapy, consequently bevacizumab is often combined with these chemotherapy backbones, with FOLFOX being the preferred front-line regimen amongst US clinicians (4,5). Contrary to these studies, other studies have suggested only modest efficacy benefit with bevacizumab. The NO16966 trial randomized, in a 2×2 factorial design, 1,401 previously untreated mCRC patients either to capecitabine and oxaliplatin (XELOX) or FOLFOX4, with bevacizumab or placebo. Despite a statistically significant improvement in progression free survival (PFS), a similar improvement in overall survival (OS) was not observed (6).
In the second-line setting, the efficacy of VEGF inhibition was demonstrated in bevacizumab-naïve patients in the ECOG 3200 trial, with significant improvements in mOS and mPFS (7). In the VELOUR trial, the novel VEGF inhibitor ziv-aflibercept with FOLFIRI after progression on first-line oxaliplatin-based regimen showed improvement in mOS (8). Results of these and other studies have been the basis for the continued prominent role of VEGF inhibition in bevacizumab-naïve mCRC patients.
Furthermore, with growing reports of rebound or flare-up of angiogenesis when VEGF-targeted therapy was withheld, clinicians favored continuing anti-angiogenic therapy after initial clinical and/or radiological progression in the first or second-line setting (9,10). This notion was supported by the TML study showing improvements in mPFS and mOS, favoring bevacizumab continuation when combined with chemotherapy backbone following progression on prior chemotherapy (11). Conversely, the GONO trial randomized mCRC patients treated first-line with bevacizumab and fluoropyrimidines (FOLFIRI, FOLFOX or FOLFOXIRI) to receive mFOLFOX6 or FOLFIRI with or without bevacizumab. Although survival data are not mature, mPFS improved from 5.2 to 6.7 months with bevacizumab [hazard ratio (HR) 0.66, P=0.0072], but mOS was 16.0 versus 16.5 months (HR: 0.83, P=0.34) (12). Despite these conflicting results and modest difference in OS, many clinicians choose to continue patients on VEGF inhibitors.
With recent FDA approval of regorafenib, an oral multikinase inhibitor with angiogenic inhibition, in patients with mCRC patients who have failed standard therapies, the continued role of anti-angiogenic therapy comes to the forefront again (13). Compared to placebo, regorafenib improved mPFS from 1.7 to 1.9 months (HR: 0.49, P<0.000001) and mOS from 5.0 to 6.4 months (HR: 0.77, P=0.005), regardless of K-RAS status (14). The real question is: does this study support the continued pivotal role of anti-angiogenic inhibitors in patients with mCRC?
Prior to regorafenib approval, mCRC patients who failed standard therapies were enrolled on phase I clinical trials. Many novel agents with various mechanisms of action have demonstrated clinical efficacy amongst patients with mCRC. However, no data on pooled efficacy data analysis are available in the literature. Our institution has been conducting early phase clinical trials for over two decades. We used our large database to identify mCRC patients enrolled into phase I studies, following bevacizumab approval and prior to regorafenib approval, to determine if VEGF inhibition continued to be beneficial after first and/or second progression. We compared the efficacy results of VEGF inhibitors versus non-VEGF targeting agents.
Materials and methods
We conducted a historical cohort analysis of mCRC patients enrolled on one of 44 phase I trials at the Institute of Drug Development at the Cancer Therapy and Research Center, University of Texas Health Science Center San Antonio, Texas, from March 2004 to September 2012. All patients were 18 years of age or older. Patients had received approved standard therapies, resulting in disease progression or unacceptable toxicity. Phase I agents were classified based on the primary mechanism of action of each drug. mPFS and mOS were estimated from Kaplan-Meier curves and groups were statistically compared with the log rank test. The magnitude of association between dichotomous factors and survival was estimated with the HR.
Results
A total of 139 patients were included in the analysis with a median age of 59 years (range, 33-81 years), 67.6% were males, 91 (65.5%) were White, 44 (31.7%) were Hispanic, three (2.2%) were African American, and one (0.7%) was American Indian. Ninety-five (68.3%) had colon cancer, and 44 (31.7%) had rectal cancer. K-RAS mutations were detected in 38.7%, and 94.9% patients had ECOG performance status of 0-1. Ninety-seven (73.9%) patients had received three or more prior chemotherapy regimens, and 89.2% had prior bevacizumab treatment with 47.7% patients receiving ten or more months of bevacizumab. No patients had received prior ziv-aflibercept or regorafenib.
The 44 phase I studies included the following classes of drugs (alone or in combination): anti-angiogenic/VEGF inhibitor-27 (19.4%), cytotoxic agents-51 (36.7%), cell cycle inhibitors-17 (12.2%), tumor microenvironment inhibitors-10 (7.2%), apoptosis/autophagy inducing agents-11 (7.9%), epidermal growth factor receptor (EGFR) inhibitors-7 (5%), growth factor inhibitors-6 (4.3%), tyrosine kinase inhibitors (TKIs)-2 (1.4%), inhibitors of protein degradation-3 (2.2%), immunologic agents-2 (1.4%), inhibitors of protein folding-2 (1.4%), and cell proliferation inhibitor-1 (0.7%). Cytotoxic agents were further subdivided into 33 (23.7%) microtubule-stabilizing agents and 18 (12.9%) DNA-damaging agents.
Reasons for patients not completing study protocol included: 112 (80.6%) disease progression, 10 (7.2%) toxicity, 13 (9.4%) self-withdrawal, and 4 (2.9%) other reasons unrelated to treatment or toxicity. The numbers of cycles completed on study were: 1 cycle—38 (27.3%), 2 cycles—56 (40.3%), 3 cycles—15 (10.8%), 4+ cycles—30 (21.6%). Patients receiving VEGF Inhibitors received, on average, 2.9 cycles, whereas those receiving non-VEGF inhibitors received an average of 2.6 cycles.
The mPFS for all 139 patients with mCRC treated on phase I trials was 2.0 months (95% CI: 1.8-2.8 months). Patients treated with VEGF inhibitors (n=27) compared to non-VEGF targeting agents (n=112) had a longer mPFS of 3.7 months (95% CI: 1.8-7.4 months) versus 1.9 months (95% CI: 1.8-2.3 months), respectively (HR: 0.60, 95% CI: 0.36-1.01, P=0.05). Nine patients were lost to follow-up and were not included in the OS analysis. The mOS for 130 patients was 6.1 months (95% CI: 5.1-6.9 months). The mOS was 6.0 (95% CI: 2.0-10.0) for patients treated with VEGF inhibitors (n=25) versus 6.2 months (95% CI: 5.1-7.0 months) for the non-VEGF targeting agents (n=105) (HR: 1.02, 95% CI: 0.64-1.63, P=0.92). Sub-group analyses were done for mPFS and mOS based on classes of agents, age, duration of prior bevacizumab therapy, and K-RAS status (Table 1).
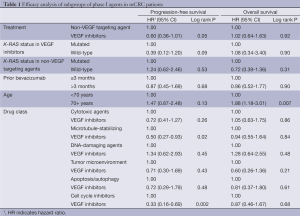
Full table
Of the 139 patients, 45 patients (32.3%) completed three or more cycles of treatment as defined by each phase I trial protocol. At 16 weeks, 19 (13.7%) patients had either stable disease (n=16) or partial response (n=3), as defined by RECIST criteria: 22% receiving VEGF inhibitors (n=6) versus 11.6% receiving non-VEGF targeting agents (n=13). For the three partial responses, treatment was with EGFR inhibitor (n=1), cytotoxic/microtubule-stabilizing agent (n=1), and growth factor inhibitor (n=1).
Treatment-related adverse events (AEs) occurred in 107 (77.0%) patients, of which 34 (24.4%) patients had grade 3-4 AEs.
Discussion
VEGF inhibition has been shown to improve PFS in mCRC in the first- and second-line settings. However, the role of VEGF inhibition is unclear after disease progression has occurred on standard agents. Prior to the approval of regorafenib, fit patients were often enrolled on phase I clinical trials. In our cohort of heavily treated mCRC patients enrolled on phase I trials after failure of standard treatments, including progression on bevacizumab, we observed a mPFS of 2.0 months and mOS of 6.1 months. Although comparison between studies should be viewed with caution, our data appears somewhat similar to the mPFS of 1.9 months and mOS of 6.2 months seen with regorafenib (14). In our cohort, we observed that patients treated with VEGF inhibitors had longer mPFS (3.7 months) compared to non-VEGF targeting agents (1.9 months). However, mOS was not statistically different (6.0 versus 6.2 months, respectively), suggesting a role for VEGF inhibition in disease stabilization. Although this did not translate to better mOS in our cohort, it mirrors clinical findings reported in some first-line and second-line studies utilizing VEGF agents (12,15). In the third-line setting, even when statistical significance is reached, as was seen with regorafenib vs. placebo, gains in PFS and OS were modest; i.e., 0.2 months (6 days) improvement in mPFS and 1.4 months (42 days) benefit in mOS (14). It is likely some patients do derive benefit from regorafenib, however, without robust predictive markers of response, the role for continued VEGF inhibition after disease progression on bevacizumab remains unclear.
In this as well as in other settings, improved PFS does not always translate to improved OS. From studies of bevacizumab in metastatic breast cancer, we have seen a reversal of FDA approval of bevacizumab, due in part to a lack of improvement in OS (16-18). This reversal raised the controversy around the inability to improve OS when powering studies for the primary endpoint of tumor response and PFS rather than OS (19). However, even with a statistically significant positive trial, such as with regorafenib, the absolute benefit in OS may be outweighed by the cost and toxicity of treatment. Thus, along with efficacy, cost and absolute differences in survival should play a role in the FDA approval of new agents.
In our cohort, we did not detect any predictive factors that would identify patients benefiting from VEGF inhibition. Our analysis showed that K-RAS status and duration of prior bevacizumab therapy did not affect efficacy outcomes. If mCRC patients who would benefit from VEGF inhibition could be identified by predictive biomarkers, treatment would become more efficacious and cost-effective. Recently, the AVAGAST trial demonstrated that plasma VEGF-A and tumor neuropilin-1 predict clinical outcome in patients with advanced gastric cancer treated with bevacizumab (20). For mCRC patients receiving bevacizumab, low levels of baseline angiopoetin-2, a key regulator of vascular remodeling in conjunction with VEGF, has been associated with better survival (21,22). Appropriate predictive biomarkers should be incorporated prospectively into early phase clinical trials in order to identify a subset of mCRC patients who would benefit from VEGF inhibition.
Our study is limited by having a heterogeneous population that was not randomized nor controlled between the two comparative groups; however, this retrospective analysis demonstrates the need to evaluate new agents in mCRC and to look beyond VEGF inhibition.
Acknowledgements
Nicole Jaime, MPH for designing the database for data collection. Subrata Haldar, PhD for help with scientific writing.
Disclosure: Devalingam Mahalingam is Advisory Board/Speaker Bureau for Bayer Pharmaceuticals. The authors declare no conflict of interest.
References
- Benson AB 3rd, Bekaii-Saab T, Chan E, et al. Metastatic colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013;11:141-52. [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [PubMed]
- Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004;22:23-30. [PubMed]
- Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229-37. [PubMed]
- Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol 2005;23:4866-75. [PubMed]
- Cassidy J, Clarke S, Diaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 2008;26:2006-12. [PubMed]
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539-44. [PubMed]
- Ruff P, Lakomy R, Prausová J, et al. Analysis of overall survival and safety during the course of the phase III VELOUR trial comparing FOLFIRI and aflibercept or placebo in patients with mCRC that progressed on prior oxaliplatin treatment. J Clin Oncol 2012;30:abstr 451.
- Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 2006;116:2610-21. [PubMed]
- Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 2007;11:83-95. [PubMed]
- Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 2013;14:29-37. [PubMed]
- Masi G, Loupakis F, Salvatore L, et al. Second-line chemotherapy (CT) with or without bevacizumab (BV) in metastatic colorectal cancer (mCRC) patients (pts) who progressed to a first-line treatment containing BV: Updated results of the phase III “BEBYP” trial by the Gruppo Oncologico Nord Ovest (GONO). J Clin Oncol 2013;31:abstr 3615.
- Bayer Health Care Pharmaceuticals. 2012. Package Insert: STIVARGA (regorafenib) tablets, oral [online]. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203085lbl.pdf2013 [Accessed 17 July 2013].
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [PubMed]
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9. [PubMed]
- Gray R, Bhattacharya S, Bowden C, et al. Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol 2009;27:4966-72. [PubMed]
- Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007;357:2666-76. [PubMed]
- Robert NJ, Diéras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 2011;29:1252-60. [PubMed]
- Cortés J, Calvo E, González-Martín A, et al. Progress against solid tumors in danger: the metastatic breast cancer example. J Clin Oncol 2012;30:3444-7. [PubMed]
- Van Cutsem E, de Haas S, Kang YK, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119-27. [PubMed]
- Goede V, Coutelle O, Neuneier J, et al. Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. Br J Cancer 2010;103:1407-14. [PubMed]
- Liu Y, Starr MD, Bulusu A, et al. Correlation of angiogenic biomarker signatures with clinical outcomes in metastatic colorectal cancer patients receiving capecitabine, oxaliplatin, and bevacizumab. Cancer Med 2013;2:234-42. [PubMed]


