Forthcoming prognostic markers for esophageal cancer: a systematic review and meta-analysis
Introduction
The incidence and mortality from cancer of all types in the United States has decreased during the 1991-2006 timeframe (1). However, the opposite is true for esophageal cancer. Its incidence and mortality continue to rise. In 2010, estimated new cases of esophageal cancer number 16,640 in the United States, while deaths total 14,500 (1). The United States has seen an average increase of 20.6% per year in the incidence of adenocarcinoma of the esophagus since that time (2). It is projected that there will be 16,470 new patients diagnosed with esophageal cancer and 14,280 deaths from it in 2008 (1).
Esophageal cancer is a highly lethal disease in which only one-third of patients present with resectable disease. Of this select group, the average 5-year survival is only 35-45% (3). Another consideration is our less-than-satisfactory ability to predict particular tumour’s response to neoadjuvant therapy. Targeted molecular therapy in upper gastrointestinal cancer has become an increasingly popular topic over the past few years. In part, this is due to rapid advances in our capability to characterize tumour biology. In esophageal cancer, VEGF (4), E-cadherin (5), COX2 (6), Survivin (7), EGFR (8) and HER2 (9) have been thoroughly investigated in the past with the help of a meta-analysis. However, insulin-like growth factor axis (IGF axis), oestrogen receptors (ER), MET or MNNG HOS Transforming gene (c-Met), octamer-binding transcription factor 4 (OCT4) and sex determining region Y-box 2 (SOX2) have not been examined.
Current concepts suggest that centrally deposited fat, so-called visceral adipose tissue, is more metabolically active than peripheral subcutaneous fat, and a more significant fuel for the association with dysmetabolism and related problems, including cancer (10). The IGF axis is thought to play a role in the link between obesity and cancer (11). The observation that insulin resistance is associated with an increased risk of cancer has led to the hypothesis that this may be mediated through the IGF axis (12,13).
One promising subset may include tumours with MET gene amplification resulting in overexpression and constitutive activation of the encoded receptor tyrosine kinase MET (14,15). In a large-scale preclinical screening approach, previously MET amplification in approximately 20% of gastric cancer cell lines and have demonstrated that this amplification confers extraordinary susceptibility to apoptosis induction by the selective MET inhibitor PHA-665752 (Pfizer, La Jolla, CA) (16). Recently, crizotinib (PF-02341066, Pfizer) was identified as an orally bioavailable, potent, ATP-competitive small-molecular inhibitor of the catalytic activity of MET kinase (17,18).
Sox2 is an important member of the Sox gene family. Sox (SRY box) genes have been identified through their homology to the high mobility group (HMG) box (79 amino acids) of sex-determining factor SRY (19-22). The Sox genes encode transcription factors that interact with DNA through their highly conserved HMG domain (23,24). The Sox genes are expressed in a wide variety of tissues, and play important roles in the regulation of organ development and cell type specification (20,22). It has been found that amplification at the chromosomal region 3q26 occurs frequently in esophageal squamous cell carcinoma (ESCC) and that SOX2 within the 3q26 amplicon is amplified and overexpressed (25).
OCT4, also known as OCT3, belongs to the POU (Pit-Oct-Unc) transcription factor family (26). The POU family of transcription factors can activate the expression of their target genes through binding the octameric sequence motif with an AGTCAAAT consensus sequence (27,28). The expression of this gene is necessary for the maintenance of pluripotentiality in embryonic stem cells (ESCs) and primordial germ cells and is down-regulated in all differentiated cells in vitro as well as in vivo (28).
The striking 3-4: one male predominance of ESCC has been observed in areas (29,30). The molecular mechanisms for such distinct gender difference in term of mortality rate and prognosis are not clear. Sex hormones, especially the typical type of oestradiol/oestrogen, and their respective receptors have been speculated to be crucial determinants for sex-related susceptibility to cancer. Oestrogen and progesterone receptors (ER and PR) are over-expressed in EC tissue whereas absent in mature normal esophageal mucosa of the foetus (31). Inhibitory effect by oestrogen on ESCC growth and development has been observed in mouse ESCC model (32). Studies on breast and endometrial cancers have shown that there are two different isoforms of human ER, i.e., ERα and ERβ, both of which are receptors for oestradiol. Recent studies have indicated that ERα expression is an unfavourable prognostic indicator in ESCC (33).
The aim of this meta-analysis was to summarize these five molecular mechanisms of disease progression, which are related to prognosis.
Methods
Study protocol
We followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses PRISMA guidelines where possible in performing our systematic review (34). We performed a systematic search through MEDLINE (from 1950), PubMed (from 1946), EMBASE (from 1949), Current Contents Connect (from 1998), Cochrane library, Google scholar, Science Direct, and Web of Science to May 2013. The search terms included “esophageal cancer”, “SOX2, OCT4, MET, IGF and oestrogen”, which were searched as text word and as exploded medical subject headings where possible. No language restrictions were used in either the search or study selection. The reference lists of relevant articles were also searched for appropriate studies. A search for unpublished literature was not performed.
Study selection
We included studies that met the following inclusion criteria:
- Studies identifying the population of patients with esophageal cancer;
- Studies dealing with the association between SOX2, OCT4, MET, insulin like growth factor receptor and oestrogen with esophageal cancer.
Data extraction
We performed the data extraction using a standardized data extraction form, collecting information on the publication year, study design, number of cases, total sample size, population type, country, continent, mean age and clinical data. The event rate and confidence intervals were calculated.
Statistical analysis
Pooled event rate and 95% confidence intervals were using a random effects model (35). We tested heterogeneity with Cochran’s Q statistic, with P<0.10 indicating heterogeneity, and quantified the degree of heterogeneity using the I2 statistic, which represents the percentage of the total variability across studies which is due to heterogeneity. I2 values of 25%, 50% and 75% corresponded to low, moderate and high degrees of heterogeneity respectively (36). The quantified publication bias using the Egger’s regression model (37), with the effect of bias assessed using the fail-safe number method. The fail-safe number was the number of studies that we would need to have missed for our observed result to be nullified to statistical non-significance at the P<0.05 level. Publication bias is generally regarded as a concern if the fail-safe number is less than 5n+10, with n being the number of studies included in the meta-analysis (38). All analyses were performed with Comprehensive Meta-analysis (version 2.0).
Results
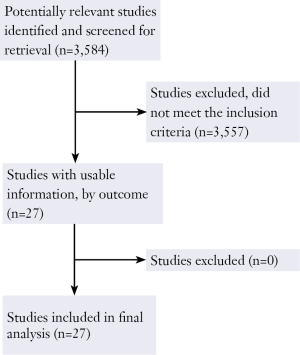
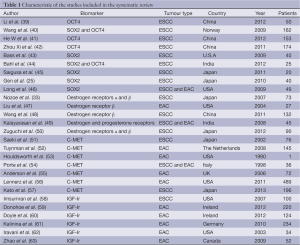
The original search strategy 3,584 retrieved studies (Figure 1). The abstracts were reviewed and articles were selected for full-text evaluation. Of the articles selected, only 27 studies (12,484 patients) met full criteria for analysis and are summarised in Table 1. This included five OCT4 studies (564 patients), six SOX2 studies (336 patients), five oestrogen receptor studies (367 patients), seven MET studies (1,015 patients) and 6 Insulin like growth factor receptor studies (764 patients). The years of publication ranged from 1990 to 2012.
Full table
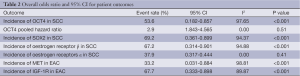
The incidence of OCT4 in SCC was 53.60% (95% CI: 0.182-0.857) and the overall hazard ratio for poor clinic outcome was 2.9 (95% CI: 1.843-4.565). The incidence of SOX2 in SCC was 69.2% (95% CI: 0.361-0.899) however, was associated with significant heterogeneity of 90.94%. The prevalence of ER α and β in SCC were 37.90% (95% CI: 0.317-0.444) and 67.20% (95% CI: 0.314-0.901) respectively. The prevalence of MET in EAC was 33.20% (95% CI: 0.031-0.884) and the incidence of insulin-like growth factor-1 receptor (IGF-1R) in EAC was 67.70% (95% CI: 0.333-0.898).
Heterogeneity and publication bias
The heterogeneity of outcomes has been summarized in Table 2. The reason for significant heterogeneity may be attributed to different population groups. No publication bias was detected using the Egger’s regression model.
Discussion
Esophageal cancer is one of the most aggressive neoplasms and the overall prognosis for esophageal cancer patients is poor (64). One of the reasons for the low survival rate is the tumour’s intrinsic resistance to many clinical therapies, especially chemotherapy. Chemotherapy often removes the bulk of a tumour mass without preventing tumour recurrence, suggesting the survival of a subset of cancer stem cells. Studies have provided experimental evidence for the concept that human tumour growth may depend on a small portion cancer stem cells (65).
SOX2 and OCT4
The expressions of Oct3/4 and Sox2 were firstly discovered in human esophageal squamous cancer cell lines with the antibody AF1759 and MAB2018 from R&D System for immunocytochemistry. Among 153 specimens from the department of Oncology at Zhengzhou University (66), 105 (68.7%) were negative or weakly positive for OCT4 staining; 21 (13.7%) were moderately positive and 27 (17.6%) were strongly positive. Higher expression level of OCT4 was significantly associated with higher histological grade (P<0.001), indicating its correlation with dedifferentiation of these tumours. The median follow-up time for the 56 patients still alive was 124 months (range, 118-155 months) and for the remaining 97 patients who died during the follow-up period was 61 months (range, 1-139 months). In univariate analysis, patients with low OCT4 expression level in tumours had a better overall survival than patients with tumour showing moderate or high OCT4 expression level (P=0.002 and Pet al. (42) Oct4 protein was expressed in most (93.7%) ESCC samples but it was not observed in esophageal mucosa. The over-expression of Oct4 in ESCCs suggests that it is a potential target for ESCC therapy. Oct4 could be a useful tumour marker in an immunohistochemical panel designed to differentiate between ESCC and esophageal mucosa. Expression of Oct4 in tumorospheres might indicate the presence of a population of ECSCs and its expression in xenograft tumours suggests that Oct4 is also associated with tumour metastasis. SOX2 gene is an amplification target of 3q26.3 in ESCC, and that SOX2 promotes ESCC cell proliferation in vitro (25). LY294002, an inhibitor of phosphatidylinositol 3-kinase, and rapamycin, an inhibitor of mTORC1, suppressed the ability of SOX2 to enhance proliferation of ESCC cells in vitro. Effects of SOX2 knockdown, including reduced levels of phosphorylated AKT and decreased ESCC cell proliferation, were reversed with constitutive activation of AKT with knockdown of phosphatase and tensin homolog. In mouse xenografts, SOX2 promoted in vivo tumor growth of ESCC, which was dependent on AKT/mTORC1 activation. LY294002 suppressed the ability of SOX2 to enhance tumor growth of ESCC by reducing cell proliferation, but not by enhancing apoptosis. These findings suggest that SOX2 promotes in vivo tumor growth of ESCC through activation of the AKT/mTORC1 signaling pathway, which enhances cell proliferation (67).
Wang et al. (40) established that Sox2 expressions were significantly associated with higher histological grade (P<0.001 for both factors), indicating their correlation to dedifferentiation in these tumours and a significant correlation between increasing levels of Sox2 immunostaining and decreasing survival for the patients (P<0.001) was observed. After being stratified by histological grade, Sox2 expressions were still significantly associated with unfavourable overall survival (P=0.008 and P=0.003, respectively).
The role of OCT4 & Sox2 in esophageal carcinogenesis evidences further studies.
Oestrogen receptor
Oestrogens, the primary female sex hormones, are mechanistically linked to aspects of cancer risk and cancer development. A connection between oestrogen-activated signalling and carcinogenesis in many organs, including mammary glands (68), ovaries and colon (69) has been clearly defined, although it is unclear whether a similar connection exists for the esophagus, and esophageal adenocarcinoma in particular. Furthermore, oestrogen is actively involved in the regulation of metabolism in adipose tissues (70), and it can be synthesized locally by activated aromatase in adipocytes in both men and women (71). Therefore it seems reasonable to consider that oestrogens might contribute towards the gender difference for esophageal adenocarcinoma. Involvement of oestrogen signalling in regulation of adipose tissue metabolism indicates a possible connection between the effects of oestrogen and male obesity-one of the main risk factors for esophageal adenocarcinoma.
A recent article from Japan (50) ERα immunoreactivity was detected in the nuclei of carcinoma cells in 38/90 ESCC ERβ immunoreactivity was detected in the nuclei of carcinoma cells with a variety of immunointensity in 88/90 ESCC. Correlation between the status of ERβ immunoreactivity and clinicopathological variables in 90 ESCC patients There was a statistically significant positive association between ERβ H score and tumor differentiation (P=0.0403) and TNM-pM (LYM) (P=0.0164). There was also a weak but statistically significant positive correlation between the ERβ H score and Ki67/MIB1 LI (P=0.0497, r=0.207). No significant association was detected between ERβ immunoreactivity and age, gender, tumor size, depth of tumor invasion, presence of lymph node metastasis, TNM stage, lymphatic invasion, venous invasion or infiltrative growth pattern of the patients examined in the present study.
The patients with positive nuclear ERα immunoreactivity in carcinoma cells were by no means associated with better survival or favorable clinical outcome (log-rank test: OS, P=0.4660; DFS, P=0.3468). In the present study, the patients with high nuclear ERβ immunoreactivity were significantly associated with shorter survival or adverse clinical outcome (log-rank test: OS, P=0.0017; DFS, P=0.0005). Results of univariate analysis (Table 2) demonstrated that pathological stage (OS, P=0.0003; DFS, P=0.0006), ERβ status in the nucleus of carcinoma cells (OS, P=0.0025; DFS, P=0.0010), tumor size (OS, P=0.0485; DFS, P=0.0366) and infiltration type (OS, P=0.0200; DFS, P=0.0416) were all significant prognostic factors for OS and/or DFS in 90 ESCC examined in our study. A subsequent multivariate analysis did reveal that ERβ status (OS, P=0.0010; DFS, P=0.0007) was an independent prognostic factor for OS and DFS of these patients, as well as pathological stage (OS, P=0.0019; DFS, P=0.0091) and infiltration type (OS, P=0.0185; DFS, P=0.0328).
Full table
Future perspective would be if a confirmed link might provide support for ERβ to be used as a target for therapy, or as a prognostic marker.
Met expression and esophageal adenocarcinoma
The Met receptor is a tyrosine kinase receptor, the product of a proto-oncogene (72). It acts as a receptor for hepatocyte growth factor (HGF), a potent mitogen and pro-motility agent for epithelial cells (73,74). HGF is primarily produced by mesenchymal cells to act on Met-expressing epithelial cells in a paracrine fashion (75).
The predominant adhesion protein of epithelial tissue is E-cadherin (13), and this is down-regulated in esophageal cancer (76). E-cadherin binds to β-catenin at the cell membrane and is linked to the control of β-catenin—regulated transcription (77,78). The β-catenin protein is found in three cellular pools: membranous, cytoplasmic, and nuclear. The translocation among these is tightly regulated (79), and the dynamic equilibrium determines the signaling role (80). Nuclear β-catenin is seen in esophageal tumorigenesis (81), and many catenin target genes show increased expression (82,83). Studies have shown an association between HGF/Met stimulation and increased phosphorylation of β-catenin in cell lines (84-86).
Studies of the expression of Met in esophageal malignancy showed increased expression in tumors compared with normal mucosa (51,77,87). Met activation in esophageal cancer induces changes consistent with early invasion, such as down-regulation of E-cadherin, increased nuclear TCF/β-catenin signaling, and anchorage-independent growth. The expression of Met in esophageal adenocarcinoma is associated with a poorer prognosis in vivo (55).
The crizotinib expanded phase I cohort study was performed by Massachusetts General Hospital/Harvard Medical School (56). Ten (2%) of 489 patients screened harbored MET amplification; 23 (4.7%) harbored EGFR amplification; 45 (8.9%) harbored HER2 amplification; and 411 (84%) were wild type for all three genes (i.e., negative). MET-amplified tumors were typically high-grade adenocarcinomas that presented at advanced stages (5%; n=4 of 80). EGFR-amplified tumors showed the highest fraction of squamous cell carcinoma (17%; n=4 of 23). HER2, MET, and EGFR amplification were, with one exception (MET and EGFR positive), mutually exclusive events. Survival analysis in patients with stages III and IV disease showed substantially shorter median survival in MET/EGFR-amplified groups, with a rank order for all groups by median survival (from most to least aggressive): MET (7.1 months; P<0.001) less than EGFR (11.2 months; P=0.16) less than HER2 (16.9 months; P=0.89) when compared with the negative group (16.2 months). Two of four patients with MET-amplified tumors treated with crizotinib experienced tumor shrinkage (-30% and -16%) and experienced progression after 3.7 and 3.5 months. MET amplification defines a small and aggressive subset of GEC with indications of transient sensitivity to the targeted MET inhibitor crizotinib (PF-02341066).
These efforts suggest that implementation of larger-scale, genome-wide assays—which would include assessment of MET copy number as well as other infrequent gene amplifications—may be an effective approach to identify multiple rare subgroups that might benefit from targeted therapies.
Insulin like growth factor axis and esophageal adenocarcinoma
Insulin resistance leads to reduced levels of IGF binding proteins and results in a subsequent increase in free IGF-1 (88). Prospective studies have shown a relationship between circulating IGF-1 and the risk of developing prostate, breast, colorectal and other cancers (12). The IGF-1R plays a role in the establishment and maintenance of cellular transformation (89), and the receptor or its ligands may be overexpressed in human tumours (90,91). Its action may protect against apoptosis, and favours invasion and metastasis (92,93).
Howard et al. (94) stated that 91% of patients with esophageal adenocarcinoma expressed leptin receptor (ObR), 95% expressed adiponectin receptors 1 (AdipR1) and 100% expressed adiponectin receptors 2 (AdipR2). Relative expression of ObR was upregulated in 67%, and AdipR1 and AdipR2 were downregulated in 55% and 68% respectively, relative to the calibrator sample. Upregulated ObR and AdipR2 expression was significantly associated with anthropometric and radiological measures of obesity. Upregulated ObR was associated with advanced tumour and node category (P=0.036 and P=0.025, respectively), and upregulated AdipR2 with nodal involvement (P=0.037).
Studies in vitro support a role for the IGF axis in esophageal adenocarcinoma progression. Blockade of the IGF-1R leads to apoptosis (95) and IGF-1 stimulates proliferation (62). In esophageal cancer, overexpression of IGF-1R has been associated with the malignant progression of Barrett’s esophagus to adenocarcinoma (96).
Trinity College (60) reported that higher IGF-1R protein expressions were observed in SCC cells compared with esophageal adenocarcinoma cells however only adenocarcinoma cell lines significantly increased proliferation in response to IGF-1 (P<0.01). Serum IGF-1 levels were highest in esophageal adenocarcinoma patients (P<0.01) and higher in viscerally obese vs. nonobese (P<0.05) patients. In resected esophageal cancer, increased expression of IGF-1R was observed in the tumor and invasive edge compared with tumor associated stroma (P<0.05), which coincided with increased CD68+ cells in stromal tissue surrounding invasive tumor edge (P<0.01).
A total of 220 patients were studied by Donohoe et al. (59). Total and free IGF-1 levels were significantly increased in the serum of viscerally obese patients. Gene expression analysis revealed a significant association between obesity status and both IGF-1R (P=0.021) and IGF-1 (P=0.031) in tumours. TMA analysis demonstrated that IGF-1R expression in resected tumours was significantly higher in viscerally obese patients than in those of normal weight (P=0.023). Disease-specific survival was longer in patients with negative IGF-1R expression than in those with IGF-1R-positive tumours (median 60.0 versus 23.4 months; P=0.027). This highlights the relationship between IGF axis with visceral obesity, and a probable impact on the biology of esophageal adenocarcinoma through its receptor.
Studies are ongoing with other novel agents targeting insulin like growth factor receptor, its ligand IGF-1, and telomerase enzyme (97).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Bollschweiler E, Wolfgarten E, Gutschow C, et al. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer 2001;92:549-55. [PubMed]
- Thompson SK, Ruszkiewicz AR, Jamieson GG, et al. Improving the accuracy of TNM staging in esophageal cancer: a pathological review of resected specimens. Ann Surg Oncol 2008;15:3447-58. [PubMed]
- Chen M, Cai E, Huang J, et al. Prognostic value of vascular endothelial growth factor expression in patients with esophageal cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2012;21:1126-34. [PubMed]
- Xu XL, Ling ZQ, Chen SZ, et al. The impact of E-cadherin expression on the prognosis of esophageal cancer: a meta-analysis. Dis Esophagus 2014;27:79-86. [PubMed]
- Li L, Zhao J, Wu Z, et al. Meta-analysis: clinicopathological and prognostic significance of cyclooxygenase-2 expression on oesophageal squamous cell carcinoma. Aliment Pharmacol Ther 2009;30:589-96. [PubMed]
- Li C, Li Z, Zhu M, et al. Clinicopathological and prognostic significance of survivin over-expression in patients with esophageal squamous cell carcinoma: a meta-analysis. PLoS One 2012;7:e44764. [PubMed]
- Yu WW, Guo YM, Zhu M, et al. Clinicopathological and prognostic significance of EGFR over-expression in esophageal squamous cell carcinoma: a meta-analysis. Hepatogastroenterology 2011;58:426-31. [PubMed]
- Chan DS, Twine CP, Lewis WG. Systematic review and meta-analysis of the influence of HER2 expression and amplification in operable oesophageal Cancer. J Gastrointest Surg 2012;16:1821-9. [PubMed]
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004;89:2548-56. [PubMed]
- Donohoe CL, Pidgeon GP, Lysaght J, et al. Obesity and gastrointestinal cancer. Br J Surg 2010;97:628-42. [PubMed]
- Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer 2004;4:505-18. [PubMed]
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 2008;8:915-28. [PubMed]
- Comoglio PM, Giordano S, Trusolino L. Drug development of Met inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 2008;7:504-16. [PubMed]
- Salgia R. Role of c-Met in cancer: emphasis on lung cancer. Semin Oncol 2009;36:S52-8. [PubMed]
- Smolen GA, Sordella R, Muir B, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci U S A 2006;103:2316-21. [PubMed]
- Christensen JG, Zou HY, Arango ME, et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther 2007;6:3314-22. [PubMed]
- Zou HY, Li Q, Lee JH, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res 2007;67:4408-17. [PubMed]
- Gubbay J, Collignon J, Koopman P, et al. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 1990;346:245-50. [PubMed]
- Pevny LH, Lovell-Badge R. Sox genes find their feet. Curr Opin Genet Dev 1997;7:338-44. [PubMed]
- Sinclair AH, Berta P, Palmer MS, et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 1990;346:240-4. [PubMed]
- Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res 1999;27:1409-20. [PubMed]
- Ferrari S, Harley VR, Pontiggia A, et al. SRY, like HMG1, recognizes sharp angles in DNA. EMBO J 1992;11:4497-506. [PubMed]
- Weiss MA. Floppy SOX: mutual induced fit in hmg (high-mobility group) box-DNA recognition. Mol Endocrinol 2001;15:353-62. [PubMed]
- Gen Y, Yasui K, Zen Y, et al. SOX2 identified as a target gene for the amplification at 3q26 that is frequently detected in esophageal squamous cell carcinoma. Cancer Genet Cytogenet 2010;202:82-93. [PubMed]
- Schöler HR, Ruppert S, Suzuki N, et al. New type of POU domain in germ line-specific protein Oct-4. Nature 1990;344:435-9. [PubMed]
- Schöler HR. Octamania: the POU factors in murine development. Trends Genet 1991;7:323-9. [PubMed]
- Pesce M, Schöler HR. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells 2001;19:271-8. [PubMed]
- Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol 2007;17:2-9. [PubMed]
- Ke L. Mortality and incidence trends from esophagus cancer in selected geographic areas of China circa 1970-90. Int J Cancer 2002;102:271-4. [PubMed]
- Wang LY. Estrogen and progesterone receptors in esophageal carcinoma cells. Zhonghua Zhong Liu Za Zhi 1991;13:23-5. [PubMed]
- Utsumi Y, Nakamura T, Nagasue N, et al. Role of estrogen receptors in the growth of human esophageal carcinoma. Cancer 1989;64:88-93. [PubMed]
- Nozoe T, Oyama T, Takenoyama M, et al. Significance of immunohistochemical expression of estrogen receptors alpha and beta in squamous cell carcinoma of the esophagus. Clin Cancer Res 2007;13:4046-50. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12. [PubMed]
- Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Orwin R. A fail-safe N for effect size in meta-analysis. Journal of educational statistics 1983;8:157-9.
- Li C, Yan Y, Ji W, et al. OCT4 positively regulates Survivin expression to promote cancer cell proliferation and leads to poor prognosis in esophageal squamous cell carcinoma. PLoS One 2012;7:e49693. [PubMed]
- Wang Q, He W, Lu C, et al. Oct3/4 and Sox2 are significantly associated with an unfavorable clinical outcome in human esophageal squamous cell carcinoma. Anticancer Res 2009;29:1233-41. [PubMed]
- Akin I, Kische S, Paranskaya L, et al. Predictive factors for pacemaker requirement after transcatheter aortic valve implantation. BMC Cardiovasc Disord 2012;12:87. [PubMed]
- Zhou X, Huang GR, Hu P. Over-expression of Oct4 in human esophageal squamous cell carcinoma. Mol Cells 2011;32:39-45. [PubMed]
- Bass AJ, Watanabe H, Mermel CH, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet 2009;41:1238-42. [PubMed]
- Bahl K, Saraya A, Sharma R. Increased levels of circulating and tissue mRNAs of Oct-4, Sox-2, Bmi-1 and nanog is ESCC patients: potential Tool for minimally invasive cancer diagnosis. Biomark Insights 2012;7:27-37. [PubMed]
- Saigusa S, Mohri Y, Ohi M, et al. Podoplanin and SOX2 expression in esophageal squamous cell carcinoma after neoadjuvant chemo-radiotherapy. Oncol Rep 2011;26:1069-74. [PubMed]
- Long KB, Hornick JL. SOX2 is highly expressed in squamous cell carcinomas of the gastrointestinal tract. Hum Pathol 2009;40:1768-73. [PubMed]
- Liu L, Chirala M, Younes M. Expression of estrogen receptor-beta isoforms in Barrett’s metaplasia, dysplasia and esophageal adenocarcinoma. Anticancer Res 2004;24:2919-24. [PubMed]
- Wang QM, Qi YJ, Jiang Q, et al. Relevance of serum estradiol and estrogen receptor beta expression from a high-incidence area for esophageal squamous cell carcinoma in China. Med Oncol 2011;28:188-93. [PubMed]
- Kalayarasan R, Ananthakrishnan N, Kate V, et al. Estrogen and progesterone receptors in esophageal carcinoma. Dis Esophagus 2008;21:298-303. [PubMed]
- Zuguchi M, Miki Y, Onodera Y, et al. Estrogen receptor α and β in esophageal squamous cell carcinoma. Cancer Sci 2012;103:1348-55. [PubMed]
- Saeki H, Oda S, Kawaguchi H, et al. Concurrent overexpression of Ets-1 and c-Met correlates with a phenotype of high cellular motility in human esophageal cancer. Int J Cancer 2002;98:8-13. [PubMed]
- Tuynman JB, Lagarde SM, Ten Kate FJ, et al. Met expression is an independent prognostic risk factor in patients with oesophageal adenocarcinoma. Br J Cancer 2008;98:1102-8. [PubMed]
- Houldsworth J, Cordon-Cardo C, Ladanyi M, et al. Gene amplification in gastric and esophageal adenocarcinomas. Cancer Res 1990;50:6417-22. [PubMed]
- Porte H, Triboulet JP, Kotelevets L, et al. Overexpression of stromelysin-3, BM-40/SPARC, and Met genes in human esophageal carcinoma: implications for prognosis. Clin Cancer Res 1998;4:1375-82. [PubMed]
- Anderson MR, Harrison R, Atherfold PA, et al. Met receptor signaling: a key effector in esophageal adenocarcinoma. Clin Cancer Res 2006;12:5936-43. [PubMed]
- Lennerz JK, Kwak EL, Ackerman A, et al. Met amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol 2011;29:4803-10. [PubMed]
- Kato H, Arao T, Matsumoto K, et al. Gene amplification of EGFR, HER2, FGFR2 and Met in esophageal squamous cell carcinoma. Int J Oncol 2013;42:1151-8. [PubMed]
- Imsumran A, Adachi Y, Yamamoto H, et al. Insulin-like growth factor-I receptor as a marker for prognosis and a therapeutic target in human esophageal squamous cell carcinoma. Carcinogenesis 2007;28:947-56. [PubMed]
- Donohoe CL, Doyle SL, Mcgarrigle S, et al. Role of the insulin-like growth factor 1 axis and visceral adiposity in oesophageal adenocarcinoma. Br J Surg 2012;99:387-96. [PubMed]
- Doyle SL, Donohoe CL, Finn SP, et al. IGF-1 and its receptor in esophageal cancer: association with adenocarcinoma and visceral obesity. Am J Gastroenterol 2012;107:196-204. [PubMed]
- Kalinina T, Bockhorn M, Kaifi JT, et al. Insulin-like growth factor-1 receptor as a novel prognostic marker and its implication as a cotarget in the treatment of human adenocarcinoma of the esophagus. Int J Cancer 2010;127:1931-40. [PubMed]
- Iravani S, Zhang HQ, Yuan ZQ, et al. Modification of insulin-like growth factor 1 receptor, c-Src, and Bcl-XL protein expression during the progression of Barrett’s neoplasia. Hum Pathol 2003;34:975-82. [PubMed]
- Zhao R, Macdonald K, Casson AG. Insulin-like growth factor type I receptor gene expression and obesity in esophageal adenocarcinoma. Mol Carcinog 2009;48:982-8. [PubMed]
- Lin YC, Wu MY, Li DR, et al. Prognostic and clinicopathological features of E-cadherin, alpha-catenin, beta-catenin, gamma-catenin and cyclin D1 expression in human esophageal squamous cell carcinoma. World J Gastroenterol 2004;10:3235-9. [PubMed]
- Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105-11. [PubMed]
- He W, Li K, Wang F, et al. Expression of OCT4 in human esophageal squamous cell carcinoma is significantly associated with poorer prognosis. World J Gastroenterol 2012;18:712-9. [PubMed]
- Gen Y, Yasui K, Nishikawa T, et al. SOX2 promotes tumor growth of esophageal squamous cell carcinoma through the AKT/mammalian target of rapamycin complex 1 signaling pathway. Cancer Sci 2013;104:810-6. [PubMed]
- Russo J, Russo IH. Breast development, hormones and cancer. Adv Exp Med Biol 2008;630:52-6. [PubMed]
- Chen JQ, Brown TR, Yager JD. Mechanisms of hormone carcinogenesis: evolution of views, role of mitochondria. Adv Exp Med Biol 2008;630:1-18. [PubMed]
- Rose DP, Vona-Davis L. Interaction between menopausal status and obesity in affecting breast cancer risk. Maturitas 2010;66:33-8. [PubMed]
- Sharpe RM. The roles of oestrogen in the male. Trends Endocrinol Metab 1998;9:371-7. [PubMed]
- Cooper CS, Park M, Blair DG, et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 1984;311:29-33. [PubMed]
- Stoker M, Perryman M. An epithelial scatter factor released by embryo fibroblasts. J Cell Sci 1985;77:209-23. [PubMed]
- Nakamura T, Teramoto H, Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci U S A 1986;83:6489-93. [PubMed]
- Maulik G, Shrikhande A, Kijima T, et al. Role of the hepatocyte growth factor receptor, c-Met, in oncogenesis and potential for therapeutic inhibition. Cytokine Growth Factor Rev 2002;13:41-59. [PubMed]
- Sanders DS, Bruton R, Darnton SJ, et al. Sequential changes in cadherin-catenin expression associated with the progression and heterogeneity of primary oesophageal squamous carcinoma. Int J Cancer 1998;79:573-9. [PubMed]
- Gottardi CJ, Wong E, Gumbiner BM. E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. J Cell Biol 2001;153:1049-60. [PubMed]
- Stockinger A, Eger A, Wolf J, et al. E-cadherin regulates cell growth by modulating proliferation-dependent beta-catenin transcriptional activity. J Cell Biol 2001;154:1185-96. [PubMed]
- Stewart DB, Nelson WJ. Identification of four distinct pools of catenins in mammalian cells and transformation-dependent changes in catenin distributions among these pools. J Biol Chem 1997;272:29652-62. [PubMed]
- Barker N, Morin PJ, Clevers H. The Yin-Yang of TCF/beta-catenin signaling. Adv Cancer Res 2000;77:1-24. [PubMed]
- Bailey T, Biddlestone L, Shepherd N, et al. Altered cadherin and catenin complexes in the Barrett’s esophagus-dysplasia-adenocarcinoma sequence: correlation with disease progression and dedifferentiation. Am J Pathol 1998;152:135-44. [PubMed]
- Arber N, Lightdale C, Rotterdam H, et al. Increased expression of the cyclin D1 gene in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev 1996;5:457-9. [PubMed]
- Morris CD, Armstrong GR, Bigley G, et al. Cyclooxygenase-2 expression in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol 2001;96:990-6. [PubMed]
- Hiscox S, Jiang WG. Association of the HGF/SF receptor, c-met, with the cell-surface adhesion molecule, E-cadherin, and catenins in human tumor cells. Biochem Biophys Res Commun 1999;261:406-11. [PubMed]
- Davies G, Jiang WG, Mason MD. Cell-cell adhesion molecules and their associated proteins in bladder cancer cells and their role in mitogen induced cell-cell dissociation and invasion. Anticancer Res 1999;19:547-52. [PubMed]
- Monga SP, Mars WM, Pediaditakis P, et al. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res 2002;62:2064-71. [PubMed]
- Herrera LJ, El-Hefnawy T, Queiroz de Oliveira PE, et al. The HGF receptor c-Met is overexpressed in esophageal adenocarcinoma. Neoplasia 2005;7:75-84. [PubMed]
- Lukanova A, Söderberg S, Stattin P, et al. Nonlinear relationship of insulin-like growth factor (IGF)-I and IGF-I/IGF-binding protein-3 ratio with indices of adiposity and plasma insulin concentrations (Sweden). Cancer Causes Control 2002;13:509-16. [PubMed]
- Sell C, Rubini M, Rubin R, et al. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci U S A 1993;90:11217-21. [PubMed]
- Hellawell GO, Turner GD, Davies DR, et al. Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res 2002;62:2942-50. [PubMed]
- Law JH, Habibi G, Hu K, et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res 2008;68:10238-46. [PubMed]
- Frasca F, Pandini G, Sciacca L, et al. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem 2008;114:23-37. [PubMed]
- Samani AA, Yakar S, Leroith D, et al. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev 2007;28:20-47. [PubMed]
- Howard JM, Beddy P, Ennis D, et al. Associations between leptin and adiponectin receptor upregulation, visceral obesity and tumour stage in oesophageal and junctional adenocarcinoma. Br J Surg 2010;97:1020-7. [PubMed]
- Piao W, Wang Y, Adachi Y, et al. Insulin-like growth factor-I receptor blockade by a specific tyrosine kinase inhibitor for human gastrointestinal carcinomas. Mol Cancer Ther 2008;7:1483-93. [PubMed]
- Liu YC, Leu CM, Wong FH, et al. Autocrine stimulation by insulin-like growth factor I is involved in the growth, tumorigenicity and chemoresistance of human esophageal carcinoma cells. J Biomed Sci 2002;9:665-74. [PubMed]
- Turner NC, Reis-Filho JS, Russell AM, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene 2007;26:2126-32. [PubMed]