Impact of treatment modality and number of lesions on recurrence and survival outcomes after treatment of colorectal cancer liver metastases
Introduction
The liver is the most frequent site of metastasis from colorectal cancer. In patients with resectable colorectal liver metastases (CLM), the efficacy of hepatic resection (HR) has been established. HR is associated with low peri-operative mortality and morbidity (1,2) and 5-year survival rates ranging from 25% to 58% (3-8). Traditionally, only 10-15% of patients with CLM were considered as candidates for HR (9). In recent years, various techniques including neoadjuvant chemotherapy (10-13), pre-operative portal vein embolization (9,14,15) and 2-stage resection approaches (9,16) have increased resectability rates, and thereby led to improved outcomes of patients with this malignant affliction. Nevertheless, because of unfavourable tumor location, inadequate hepatic reserve or disease extent, only 20-40% of patients with CLM will be candidates for HR in the contemporary era (13,17).
For patients who are not suitable for HR of CLM, several liver-directed therapies and adjuncts have been proposed to expand the indications for potentially curative therapy. Intraparenchymal ablative techniques, including radiofrequency ablation (RFA), microwave ablation (MWA) and cryoablation, have been widely studied (18). Unfortunately, high local recurrence rates of around 40% and the lack of long-term outcomes data have precluded the widespread adoption of these techniques (3,18,19). Many investigators have argued that ablation should only be used sparingly and restricted to patients with small lesions (3,20-23). Conversely, other investigators have proposed that ablation is effective, both as adjunct to HR or as an isolated treatment option for patients with limited hepatic involvement or solitary metastases (17,24-29). Although significant efforts made by the surgical oncology community to define the role of ablation, further studies are necessary. In particular, although studies have shown that in patients with resectable disease, the outcomes of resection compared to combined resection and ablation are similar, there is significantly less comparative data for multiple lesions. Some studies included patients with extra-hepatic disease leading to confounding of the results to evaluate each treatment modalities.
The aim of the current study was to evaluate the role of resection, combined resection and ablation and isolated ablation in the management of a large number of patients with isolated CLM. More specifically, we sought to determine the influence of treatment type on outcomes for patients with 1-4 and ≥5 lesions, respectively.
Patients and methods
We reviewed the records of 701 consecutive patients with colorectal hepatic metastases without extra-hepatic disease who underwent hepatic intervention from a prospective database. All procedures were performed at the Hepatobiliary Service of the University of New South Wales, Department of Surgery, St George Hospital between April 1990 and December 2010. All patients had previously diagnosed colorectal cancer and were treated with curative intent. Patients were evaluated with a baseline medical history, clinical examination, serum laboratory tests including the tumor marker carcinoembryonic antigen (CEA), computed tomography (CT) angiogram of the liver, whole body CT (chest, abdomen, and pelvis), and chest radiography. Patients who underwent open and close procedures without hepatic intervention for their tumor were excluded from this study.
Operative techniques
An initial laparotomy through a right sub-costal incision was made for all patients. Exploration and palpation of the liver, hilar region and the abdominal cavity were performed to determine the presence of extrahepatic disease. Any suspicious lymph nodes or peritoneal nodules were biopsied and sent for frozen section histology. Intraoperative ultrasound of the liver was performed in every patient to assess the liver metastases by identifying, counting, and characterizing the nature and vascular proximity of the metastatic lesions. When surgery was considered feasible, the incision was extended to bilateral sub-costal or triradiate incision and the liver was then fully mobilized. For liver parenchymal transection, an ultrasonic dissector (Sumisonic ME-2210; Sumitomo Bakelite Co., Japan or Selector Spembly UK) was used. Cryoablation was performed using the L.C.S. 3000 liquid nitrogen system (Spembly, Andover, UK) or the Erbe system (Tubingen, Germany). Intra-operative ultrasound was used to monitor ice-ball formation to ensure tumor clearance in all planes by a margin of at least 1 cm, and the freezing process was continued for at least 5 min. All patients were explored with an operative intent. Indications for ablations were:
- Deep seated tumours in the ipse-lateral lobe when a parenchymal sparing technique was used;
- Deep seated tumours in the contra-lateral lobe when a parenchymal sparing technique was used;
- Those patients deemed poor candidates for an open liver resection.
Postoperative management
All patients were admitted to the intensive care unit during the early postoperative period after surgery. Patients were commenced on oral intake when bowel function was regained and drain tubes were removed when output was low. Following discharge, all patients were followed prospectively at monthly intervals for the first three months and at six monthly intervals thereafter with clinical examination, CEA measurement and CT of the chest, abdomen and pelvis. Recurrence was identified by hospital radiologists after comparison with previous CT scans. Recurrence was managed based on a decision by a multidisciplinary team based on the location of recurrent disease, extent of recurrent disease and the patient’s performance.
Data collection and statistical analysis
Patient demographic data, disease-related factors, pathological factors and treatment-related factors were prospectively collected and analysed. The primary endpoints were the time from hepatic intervention to the time of disease recurrence [recurrence-free survival (RFS)] and cancer-related death (overall survival). Follow-up data was obtained from the referring physicians and phone calls and/or emails from the patients. Data analyses were performed using SPSS® for Windows version 17.0 (SPSS, Munich, Germany). The patient characteristics were reported using frequency and descriptive analyses. The Kaplan-Meier method was used to analyze progression-free survival and overall survival. Univariate analysis (log-rank) was performed to examine the survival and overall survival. The median time to death was defined as the time where 50% of patients have died. Follow-up was calculated from the date of treatment of colorectal cancer liver metastases to the date of death or last follow-up. P<0.05 was considered statistically significant.
Results
A total of 701 patients (441 men and 260 women) with isolated CLM underwent surgical intervention between April 1990 and December 2010. Of these 462 (66%) patients underwent isolated HR, 148 (21%) underwent concomitant resection and ablation and 91 (13%) underwent isolated ablation. Patient demographics and treatment-related factors for all patients are summarized in Table 1.
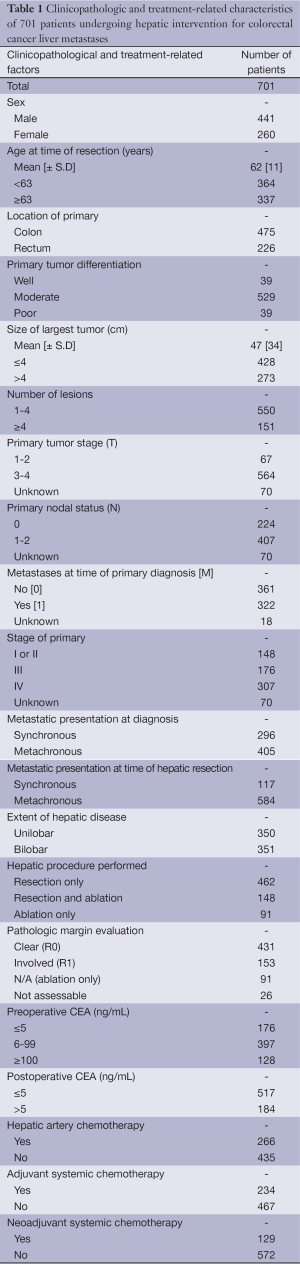
Full table
Of the 701 patients, 550 patients (78%) presented with 1-4 lesions at the time of hepatic intervention (Group A) and 151 patients (22%) presented with ≥5 lesions (Group B). Of group A patients, 403 patients (73%) underwent isolated HR, 83 patients (15%) underwent concomitant HR and ablation and 64 patients (12%) underwent isolated ablation. Of group B patients, 59 patients (39%) underwent isolated HR, 65 patients (43%) underwent concomitant HR and ablation and 27 patients (18%) underwent isolated ablation.
A comparison of 18 clinicopathologic and treatment-related characteristics of patients, according to the number of hepatic lesions and the type of hepatic intervention performed is provided in Table 2. In group A, patients who underwent isolated resection were more likely to have a rectal primary (P=0.031), largest tumor size >4 cm (P=0.026), unilobar disease (P=0.001) and less likely to have undergone hepatic artery chemotherapy (P<0.001). Patients who underwent isolated resection were also more likely to have a clear surgical margin, compared to patients who underwent concomitant resection and ablation (P=0.032). Post-operative CEA was lowest in patients who underwent concomitant resection and ablation and highest in those who underwent isolated ablation (P<0.001).
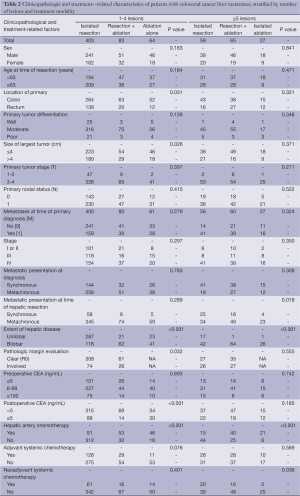
Full table
In group B, patients who underwent isolated resection were more likely to present with unilobar disease (P<0.001), synchronous disease at diagnosis (P=0.018) and undergo treatment with neoadjuvant chemotherapy (P=0.036). Conversely, they were less likely to undergo treatment with hepatic artery chemotherapy (P<0.001). There were no other significant differences between the two groups.
Survival outcomes
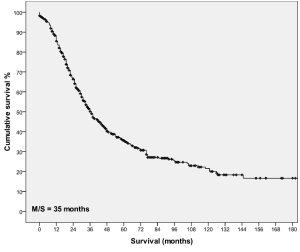
Follow-up was complete in 98% of patients. Thirteen patients (2%) died within 30 days of surgery and 460 (66%) patients died at the time of last follow-up. The median follow-up of period for the patients who were alive was 46 months (range, 1 to 187 months). The median survival after hepatic intervention for all patients was 35 months with 1-, 3-, 5-, 10-, 15-year survival of 86%, 49%, 33%, 20% and 15% respectively (Figure 1).
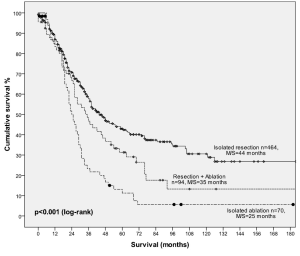
For patients with 1-4 lesions, median survival was 37 months with 1, 3-, 5-, 10-, 15-year survival of 88%, 52%, 36%, 22% and 17%, respectively. Stratified by procedure type, 5-year survival was 41% in patients who underwent isolated resection, 35% in patients who underwent concomitant resection and ablation and 13% in patients who underwent ablation alone (Figure 2). This difference was statistically significant (P<0.001).
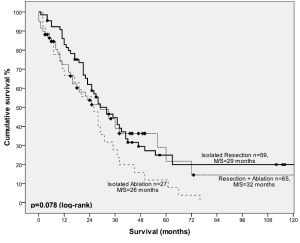
For patients with ≥5 lesions, median survival was 28 months with 1-, 3-, 5-, 10-year survival of 78%, 41%, 23% and 14% respectively. Stratified by procedure type, 5-year survival was 36% in patients who underwent isolated resection, 25% in patients who underwent concomitant resection and ablation and 12% in patients who underwent ablation alone (Figure 3). There was no statistical difference between the two groups (P=0.078).
Recurrence outcomes
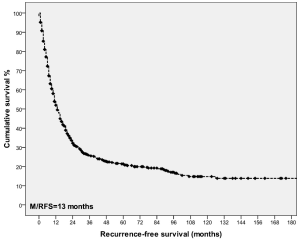
Recurrence was assessed in the 688 patients who survived beyond one month of surgery. During post-operative follow-up, 505 (73%) patients developed disease recurrence. The median time to recurrence was 13 months (range, 1-187 months). RFS after 1-, 3-, 5-, 10-year was 54%, 26%, 21% and 15%, respectively (Figure 4).
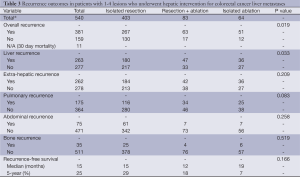
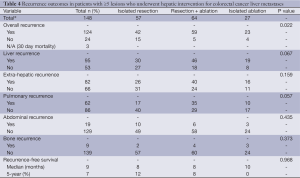
Sites of recurrence included the liver in 358 (51%) patients and extra-hepatic sites in 344 (49%); this included pulmonary recurrence (n=237; 34%), bone recurrence (n=44; 6%) and abdominal recurrence (n=94; 13%). A comparison of recurrence outcomes in patients with 1-4 lesions and ≥5 lesions is provided in Tables 3,4 respectively.
Full table
Full table
Discussion
In our institutional analysis of 701 patients, the median survival was 35 months with a 5- and 10-year survival of 33% and 20%, respectively. The results are comparable to contemporary series from major hepatobiliary centres (3-8).
For patients with 1-4 lesions, median survival 37 months and 5-year survival was 36%. When patients with 1-4 lesions were stratified by treatment type, 5-year survival was significantly superior in patients who underwent isolated resection (41%) compared to those who underwent concomitant resection and ablation (35%) or isolated ablation (13%) (P
It could be argued that patients undergoing ablation alone had a poorer performance status and hence were not offered resection and had a lower survival rate because of medical co-morbidities. It was observed that patients undergoing an ablation alone had significantly higher overall and liver specific recurrence rates. This is likely a reflection of the type of surgical technique and its impact on tumor eradication, suggesting that resection remains superior to ablation. In this regard, our data suggests that isolated resection, whenever possible, is the preferred treatment option in patients with low tumor number. Previous studies comparing resection and ablation in patients with low volume CLM have shown similar results (3,20,21,23). Aloia and colleagues (20) evaluated the outcomes of 180 patients with a solitary CLM who underwent treatment at the M.D Anderson Cancer Center; of these, 150 patients were treated with isolated resection and 30 patients with isolated ablation. The authors demonstrated that both 5-year disease-free survival (50% vs. 0%) and overall survival (71% vs. 27%) were higher in patients treated with isolated resection. This remained true even when only patients with small lesion size (≤3 cm) were included in the analysis (P<0.001). The authors concluded that every method should be employed to achieve resection of solitary CLM, including referral to a specialty center, extended hepatectomy, and chemotherapy.
A 423-patient Italian multicenter trial also demonstrated poor results in patients undergoing isolated RFA (30). Inclusion criteria were ≤4 lesions and a maximum tumor diameter of 5 cm. Despite these restrictive criteria, the overall 5-year survival was only 24%. Moreover, 5-year survival was only 11% in patients with multiple tumors and 13% in patients with solitary lesions greater than 2.5 cm in diameter. These data are comparable to the 5-year survival reported in our isolated ablation cohort.
Others have suggested ablation is rarely necessary in the management of CRCLM. Kornprat and colleagues (22), from the Memorial Sloan-Kettering Cancer Center argued that of the 669 patients who underwent treatment, only 39 patients (5.9%) underwent concomitant treatment with RFA or cryoablation. The authors advocated resection as the primary treatment option and ablation as an adjunct in patients with tumors that would be otherwise unresectable.
Whilst the majority of studies have demonstrated that HR achieves significantly better outcomes than ablation, other studies have shown comparable outcomes between the two. Oshowo and colleagues (25), reported a comparative analysis of patients with solitary CLM treated by HR or RFA. The study demonstrated a similar 3-year survival rates in the two groups (55% for HR and 53% for RFA) although no long-term survivors were documented in the RFA group. An important limitation of that series was its sample size which was small at only 45 patients and the inclusion of patients with extra-hepatic disease. Nevertheless, the authors advocated a greater role of ablation in the management of solitary liver metastases given that it is less invasive and requires a shorter hospital stay. More recently, Hammill and colleagues (27) demonstrated a 5-year survival of 49% in 64 patients treated with RFA who satisfied “resection” criteria, although resectability status was determined retrospectively. In contrast, 5-year survival was only 18% in the “unresectable” group. Overall, however, despite notable exceptions, the majority of published series support the use of HR as the primary treatment option in patients with limited disease.
Perhaps the most important role for ablation is in the management of patients with advanced, unresectable disease. Although number of lesions is no longer a definitive criterion for unresectability in the contemporary era, many clinicians remain reluctant to offer surgery to patients with high tumor number (9,13). Ablative techniques, either in isolation or combined with surgery may be appropriate in these cases, although reluctance to offer these treatments to patients with higher tumor number also exists.
Our study demonstrates encouraging outcomes in patients with ≥5 lesions independent of which treatment modality was used. The median survival was not significantly different in patients who underwent HR, combined HR and ablation or ablation alone at 29, 32 and 26 months, respectively. Similar trends have been observed elsewhere. Rivoire and colleagues (17) analyzed the outcomes of 57 patients treated with HR or combined HR and cryoablation after neoadjuvant chemotherapy. There were no major differences between the two groups and 4-year survival was similar for both HR and combined HR and ablation at 37% and 36%, respectively. Interestingly, however, 59% of patients who underwent the combined procedure had ≥5 tumors compared to 27% in the HR resection group. In this regard, concomitant HR and ablation was shown to not only achieve comparable outcomes to HR alone but effectively expand the criteria for resectability. An earlier series by Wallace and colleagues (31) evaluated the outcomes of 77 patients of which 47 underwent cryoablation. The authors showed that ablation was associated with a similar 3-year survival to HR (37% vs. 36%) and specifically allowed the surgical treatment of patients previously deemed unresectable because of number of lesions. The authors concluded that incorporating cryoablation into the armamentarium for the treatment of advanced CLM extends the indication for resection and improves outcomes. Our findings are consistent with these findings. However, it must be pointed out that for ≥5 lesions, ablation with or without a resection had a higher overall and liver specific recurrence rates.
Despite the importance of ablative strategies in the management of CLM, it is important to acknowledge other strategies which have expanded the resectability criteria of patients with advanced CLM. The introduction of new cytotoxic agents including oxaliplatin and irinotecan have increased response rate from historical 20% of 5-flurouracil up to 66% and improved the median overall survival up to 22 months (9-13). More recently, the introduction of biologic agents such as bevacizumab has improved outcomes (32). Portal vein embolization and a 2-stage hepatectomy are emerging strategies (9,14-16). Moreover, identification of novel prognostic factors incorporating response to therapy and tumor biology may optimize patient selection (9). These techniques may facilitate an increase in both the quality and quantity of patients selected for a potentially curative hepatic procedure.
In conclusion, our study suggests that ablation is an important tool in hepatic surgery. Although the outcomes of ablation in patients with limited disease (1-4 lesions) is noticeably inferior to resection alone, our data suggests that its utility in patients with ≥5 lesions is promising. Combining resection and ablation in patients with multiple and advanced CLM may expand the selection criteria for surgery and offer a curative treatment to candidates who would otherwise be offered chemotherapy only. A randomized trial comparing ablation and resection in patients with solitary CLM or limited disease may be an approach to offer minimally invasive treatment, however, our data suggests that the outcomes of surgery is likely to be superior. The utility of ablation may be more appropriate in the setting of advanced disease to serve as a tumor burden eradicating strategy to enhance the efficacy of chemotherapy. As multimodality treatment strategies for CLM continue to advance, an individualized approach based on the currently available evidence appears to be the most appropriate approach to guide management.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Vauthey JN, Pawlik TM, Abdalla EK, et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg 2004;239:722-30; discussion 730-2. [PubMed]
- Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg 2000;191:38-46. [PubMed]
- Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239:818-25; discussion 825-7. [PubMed]
- Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 2005;241:715-22, discussion 722-4. [PubMed]
- Fernandez FG, Drebin JA, Linehan DC, et al. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann Surg 2004;240:438-47; discussion 447-50 [PubMed]
- de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg 2009;250:440-8. [PubMed]
- Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996;77:1254-62. [PubMed]
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. [PubMed]
- Yang AD, Brouquet A, Vauthey JN. Extending limits of resection for metastatic colorectal cancer: risk benefit ratio. J Surg Oncol 2010;102:996-1001. [PubMed]
- Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol 2010;11:38-47. [PubMed]
- Adam R, Avisar E, Ariche A, et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol 2001;8:347-53. [PubMed]
- Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000;355:1041-7. [PubMed]
- Nordlinger B, Van Cutsem E, Gruenberger T, et al. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol 2009;20:985-92. [PubMed]
- Wicherts DA, de Haas RJ, Andreani P, et al. Impact of portal vein embolization on long-term survival of patients with primarily unresectable colorectal liver metastases. Br J Surg 2010;97:240-50. [PubMed]
- Abdalla EK. Portal vein embolization (prior to major hepatectomy) effects on regeneration, resectability, and outcome. J Surg Oncol 2010;102:960-7. [PubMed]
- Kianmanesh R, Farges O, Abdalla EK, et al. Right portal vein ligation: a new planned two-step all-surgical approach for complete resection of primary gastrointestinal tumors with multiple bilateral liver metastases. J Am Coll Surg 2003;197:164-70. [PubMed]
- Rivoire M, De Cian F, Meeus P, et al. Combination of neoadjuvant chemotherapy with cryotherapy and surgical resection for the treatment of unresectable liver metastases from colorectal carcinoma. Cancer 2002;95:2283-92. [PubMed]
- Brown RE, Martin RC 2nd, Scoggins CR. Ablative therapies for colorectal liver metastases. Surg Oncol Clin N Am 2011;20:259-71. [PubMed]
- Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology 2001;221:159-66. [PubMed]
- Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006;141:460-6; discussion 466-7. [PubMed]
- White RR, Avital I, Sofocleous CT, et al. Rates and patterns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. J Gastrointest Surg 2007;11:256-63. [PubMed]
- Kornprat P, Jarnagin WR, Dematteo RP, et al. Role of intraoperative thermoablation combined with resection in the treatment of hepatic metastasis from colorectal cancer. Arch Surg 2007;142:1087-92. [PubMed]
- Hur H, Ko YT, Min BS, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg 2009;197:728-36. [PubMed]
- Abitabile P, Hartl U, Lange J, et al. Radiofrequency ablation permits an effective treatment for colorectal liver metastasis. Eur J Surg Oncol 2007;33:67-71. [PubMed]
- Oshowo A, Gillams A, Harrison E, et al. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg 2003;90:1240-3. [PubMed]
- Livraghi T, Solbiati L, Meloni F, et al. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the “test-of-time approach”. Cancer 2003;97:3027-35. [PubMed]
- Hammill CW, Billingsley KG, Cassera MA, et al. Outcome after laparoscopic radiofrequency ablation of technically resectable colorectal liver metastases. Ann Surg Oncol 2011;18:1947-54. [PubMed]
- Yan TD, Padang R, Morris DL. Longterm results and prognostic indicators after cryotherapy and hepatic arterial chemotherapy with or without resection for colorectal liver metastases in 224 patients: longterm survival can be achieved in patients with multiple bilateral liver metastases. J Am Coll Surg 2006;202:100-11. [PubMed]
- Pawlik TM, Izzo F, Cohen DS, et al. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol 2003;10:1059-69. [PubMed]
- Lencioni R, Crocetti L, Cioni D, et al. Percutaneous radiofrequency ablation of hepatic colorectal metastases: technique, indications, results, and new promises. Invest Radiol 2004;39:689-97. [PubMed]
- Wallace JR, Christians KK, Pitt HA, et al. Cryotherapy extends the indications for treatment of colorectal liver metastases. Surgery 1999;126:766-72; discussion 772-4 [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal Cancer. N Engl J Med 2004;350:2335-42. [PubMed]


